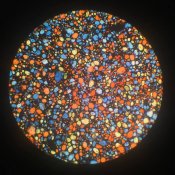
One thing you may want to research would be the proportions of each color in the mix. As the Aura film is a bit weak in the green I would use more green colored starch to offset the lack of response. I've seen how some digital cameras use a grid of 2 green to 1 red and 1 blue to optimise the color response, so this strategy has precedent.
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Autochromes...
-
A
- Thread starter htmlguru4242
- Start date
Recent Classifieds
-
For Sale Fujifilm MAXIMA Glossy RA-4 Paper; Packs of 16x20 Sheets
- Started by Aidan Sciortino
-
Sold 1924 Carl Zeiss Jena 150mm f4.5 Lens in dial Compur
- Started by Jon Shiu
-
Under Offer Plastic 5x7 film holders (Fidelity, Lisco)
- Started by blee1996
-
Want to Buy WTB: Digital timer for an enlarger
- Started by Terrence Brennan
-
Sold Fujinon SW 90mm f8 wide angle lens in Seiko shutter
- Started by Simon Benton
Forum statistics
Good suggestion. Thinking about it, I do remember reading somewhere that the original Autochromes used slightly more green starch than other colors. This seems to be prevalent in digital as wel, as you mention, althouhg as to why, I'm not sure...
I'll start with as close to the original proportions as I can get, and go from there. The only thing that I'm worried about is the incredibly high infrared and Ultraviolet sensitivity of the film. 'm not sure if this will set the color rendition off.
I'm just finishing up with some starch dyeing experiments. Once it dries, I'll let everyone know how it came out.
Well, back to stirring the starch to ensure even drying...
I'll start with as close to the original proportions as I can get, and go from there. The only thing that I'm worried about is the incredibly high infrared and Ultraviolet sensitivity of the film. 'm not sure if this will set the color rendition off.
I'm just finishing up with some starch dyeing experiments. Once it dries, I'll let everyone know how it came out.
Well, back to stirring the starch to ensure even drying...
I've dyed two colors of starch: blue and red; the red came out a bright orange, but the blue came out very well. Next is mixing it with something to coat it into a mask.
FWIW, the micrographs I've seen of the earlier autochromes had a rather orange color to the red granules as well. Since the image will be viewed through the same filter granules it was exposed with, the overall color will be untrue only if there's a significant lack of filtration in one or another region, or a lack of sensitivity in the film. That is, you need to have *some* red in one or more of the filter colors, but as I recall the original colors were more orange, green, and violet than cyan, yellow, magenta or red, green, blue. Even though the color is technically additive in this usage, you can still get good color as well as a brighter trannie and better speed by using something closer to CMY (because each grain transmits more light).
I've seen the same thing. I recall reading that the original Autochromes used two layers of orange, violet and light green granules. This way, there was little space in between granules, and there would be adequate, but not too much filtration. I'm working on the rest of the colors.
Altough the mask screen is not posing me problems, the emulsion is still going to be a trick. My idea of gluing to sheet film may work, but from a longevity point of view, might not be idea, as I do not know if anyhting will shift, expand or shink over time.
I'm still looking for a panchromatic emulsion formula, but all I've found are formulas for Red - sensitive holography emulsions (for 530nm or 630nm HeNe lasers); these are not expensive or particularly difficult to make, but lack green and yellow sensitivity, and do not appear to be very fast. They mention the usage of PInacyanol Chloride and Dicarbocyanine Iodide dyes, whihc are expensive at first, but appear to go a long way. I am not, however, familiar enough with photographic chemistry to make these emulsions panchromatic.
Does anyone know of a panchromatic film manufacturer that might be willing to send me some of thier panchromatic film emulsions in liquid form, or is anyone familiar with what dyes would panchromatically sensitize an emulsion?
I'm still looking for a panchromatic emulsion formula, but all I've found are formulas for Red - sensitive holography emulsions (for 530nm or 630nm HeNe lasers); these are not expensive or particularly difficult to make, but lack green and yellow sensitivity, and do not appear to be very fast. They mention the usage of PInacyanol Chloride and Dicarbocyanine Iodide dyes, whihc are expensive at first, but appear to go a long way. I am not, however, familiar enough with photographic chemistry to make these emulsions panchromatic.
Does anyone know of a panchromatic film manufacturer that might be willing to send me some of thier panchromatic film emulsions in liquid form, or is anyone familiar with what dyes would panchromatically sensitize an emulsion?
You will probably need two dyes to achieve panchromaticity. One will be for red and the other for green. I have not found any panchromatic dyes available on the market at the present time. In addition, I have not found any panchromatic emulsions being supplied either.
You may even need a blend of 3 emulsions, One undyed blue sensitive, and one each dyed green and red. The reason is that adding the dyes decreases blue sensitivity and can repress it enough that there is not enough blue sensitivity left to record the blue image properly in daylight.
You will have to do a dye series to determine which dye level is proper for the emulsion and dye combination. The correct level is based on the surface area of the grain and follows a Langmuir adsorption curve.
I had to buy 4 dyes to get 2 good ones, so the hidden cost in these dyes is that you have to pay for R&D and cannot just go buy one and be sure that it will work out of the bottle. Even knowing what I was doing only gave me a success rate of 50%.
To properly test your work, you will want to have access to a spectrosensitometer. This instrument puts out an equal energy spectrum of 400 - 700 nm through a step wedge, and is used to judge the senstivity and balance of the emulsion (daylight, tungsten and etc). If you wish, I can supply you with examples off-line.
Assuming you succeed, you then have the problem of coating the emulsion facing you. Doing this in total darkness, as I have said before is not trivial if you wish to have the uniformity needed for good quality. I can do the green just fine, but doing the red sensitive emulsion generally leads to problems in the dark.
I have blue and green emulsions which have ISO speeds of about 12 - 25 right now, and I have just made an emulsion with ISO 50 in the raw state. These are not easy to make or sensitize. I have about 50 grams of each made up right now. I am still testing the ISO 50 emulsion and will likely use up this batch just in testing. I have formulas for faster ones that I intend to make and which are reported to be in the range of 100 - 200.
I have, at present, had no thoughts regarding distributing these, but we can discuss this off-line if you wish. I have no tests run on the keeping characteristics of the emulsions and have no idea how well they would ship. They keep well in my refrigerator in the raw state for months, but sensitized versions are used up so rapidly in testing that I have no good data yet. Also, the proper addenda to restrain keeping fog and stabilze the emulsions are hard to get and expensive. But, they also often decrease speed, so we are on the horns of a dilemma to get optimum results and this requires tedious iterative experiments. I am in the midst of them.
PE
You may even need a blend of 3 emulsions, One undyed blue sensitive, and one each dyed green and red. The reason is that adding the dyes decreases blue sensitivity and can repress it enough that there is not enough blue sensitivity left to record the blue image properly in daylight.
You will have to do a dye series to determine which dye level is proper for the emulsion and dye combination. The correct level is based on the surface area of the grain and follows a Langmuir adsorption curve.
I had to buy 4 dyes to get 2 good ones, so the hidden cost in these dyes is that you have to pay for R&D and cannot just go buy one and be sure that it will work out of the bottle. Even knowing what I was doing only gave me a success rate of 50%.
To properly test your work, you will want to have access to a spectrosensitometer. This instrument puts out an equal energy spectrum of 400 - 700 nm through a step wedge, and is used to judge the senstivity and balance of the emulsion (daylight, tungsten and etc). If you wish, I can supply you with examples off-line.
Assuming you succeed, you then have the problem of coating the emulsion facing you. Doing this in total darkness, as I have said before is not trivial if you wish to have the uniformity needed for good quality. I can do the green just fine, but doing the red sensitive emulsion generally leads to problems in the dark.
I have blue and green emulsions which have ISO speeds of about 12 - 25 right now, and I have just made an emulsion with ISO 50 in the raw state. These are not easy to make or sensitize. I have about 50 grams of each made up right now. I am still testing the ISO 50 emulsion and will likely use up this batch just in testing. I have formulas for faster ones that I intend to make and which are reported to be in the range of 100 - 200.
I have, at present, had no thoughts regarding distributing these, but we can discuss this off-line if you wish. I have no tests run on the keeping characteristics of the emulsions and have no idea how well they would ship. They keep well in my refrigerator in the raw state for months, but sensitized versions are used up so rapidly in testing that I have no good data yet. Also, the proper addenda to restrain keeping fog and stabilze the emulsions are hard to get and expensive. But, they also often decrease speed, so we are on the horns of a dilemma to get optimum results and this requires tedious iterative experiments. I am in the midst of them.
PE
Ok, so i can now multiply what i thought would be hte difficulty of this by about 10...
It might be easier if it would be possible to find hte Lumiere's forumulas. I'm going to contact Ilford on that, as their the ones who've inherited the Lumiere plant.
It might be easier if it would be possible to find hte Lumiere's forumulas. I'm going to contact Ilford on that, as their the ones who've inherited the Lumiere plant.
htmlguru4242 said:Ok, so i can now multiply what i thought would be hte difficulty of this by about 10...
It might be easier if it would be possible to find hte Lumiere's forumulas. I'm going to contact Ilford on that, as their the ones who've inherited the Lumiere plant.
Well, it isn't going to be difficult, it is going to be time consuming and expensive. Wall, an early author of several books on emulsion making said something like this "the only thing that equals emulsion making in expense to the devoted hobbyist is dabbling in the stock market". Once all of the research is done though, it becomes reasonably economical. It is very satifying.
I doubt it you can get formulas from anyone without paying a very stiff price if you can get one at all that is useful today. Remember that Lumiere's formulas used active gelatins which are not used today. They probably used so called 'boiled' makes or ammoniated makes which are quite different than todays work. That is not to say that they are not useful, but rather that they are going to have to be 'adjusted' to work with today's gelatins. I also find that just as in todays patents, a lot of the lost art is left out of these formulas. The ones that I have seen are by no means complete or accurate.
PE
Well then, I'll put the emulsion making on hold ... for now. This weekend I'll dye the rest of the starch and coat it onto a plate, experimenting with a way to adhere it well.
I had an opportunity to talk to an expert in Autochromes today. He said that they had restored the original machine that made Autochromes. It was found in France at a location near the Lumiere home and was painstakingly restored.
As of now, several years later, they have been unable to get it to make one single piece of the color mask from starch grains. In fact, in all of the tries around the world in the last half century, he knows of no one who has been able to accomplish this critical and difficult step.
PE
As of now, several years later, they have been unable to get it to make one single piece of the color mask from starch grains. In fact, in all of the tries around the world in the last half century, he knows of no one who has been able to accomplish this critical and difficult step.
PE
Hmm, sounds as if there was a secret pretreatment done to the starch grains that was missed. Perhaps hydrolysing the starch just before or after application to the glass? Interesting info PE.
Hm ... I'm having exacty the same problem. I've dyes corn starch (easy to work with for now), and suspended them in various substances (so far honey works well, but it never dries, so off to another substance), though it sort of works. However, it is incredibly difficult to get even distribution of a monolayer.
I'm going ot try gelatine again, though I have not been able to find a dye that doesn't come off slightly in water (the grains remain colored, but diffuse some dye into the aqueous environment). I'll try washing them a few times before suspension in gelatine.
In some research, I did discover that the grains were separated by size by a process that involved floating them in water - this may be something...
Someone at <http://www.bway.net/~jscruggs/specific.html> seems to ahve figured it out, and has produced a filter plate...
Also, PE, do you ahve any contact info. for said Autochrome expert??
I'm going ot try gelatine again, though I have not been able to find a dye that doesn't come off slightly in water (the grains remain colored, but diffuse some dye into the aqueous environment). I'll try washing them a few times before suspension in gelatine.
In some research, I did discover that the grains were separated by size by a process that involved floating them in water - this may be something...
Someone at <http://www.bway.net/~jscruggs/specific.html> seems to ahve figured it out, and has produced a filter plate...
Also, PE, do you ahve any contact info. for said Autochrome expert??
Yes, please contact me for name and e-mail.
PE
PE
I've been making some progress here, so here's a status update. I have dyed starch grains red, green, blue, violet and orange. I have all of these colors should I need them.
I'm currently experimenting with ways to coat these onto the plate. I've come up with a few different ideas, with varying degrees of success:
1.) Mix with adhesive, then coat: This sort of works, but results is a coating that is far from a monolayer; on hold for now.
2.) Sprinkle [great technical term] the starch onto an adhesive: Difficult to prevent tiny grains from clumping, hard to coat an even monolayer.
3.) Brush coating: The plate is coated with an adhesive, and then the starch is lightly brushed on. This works well, though care must be exercised not to put brush marks in the adhesive base. It is definetely doable, especially if pressure is applied to the plate after the coating is done.
4.) Coat plate wiht adhesive, submerge in liquid with suspension of starch: Basically, I mix the starch with water, place the plate in a container and pour the water / starch into it. The plate is then lifted, creating a coating of starch. This works ok, but has the issue of water remaining on the plate and disturbing hte adhesive / speading the starch around. Perhaps it would work with water containing a surfactant or some other liquid (acetone or ethanol, perhaps?)
For adhesive, i've experimented with everything from honey to epoxy. Epoxy seems to work well, though it hardens a little more quickly than I'd like.
I'm currently experimenting with ways to coat these onto the plate. I've come up with a few different ideas, with varying degrees of success:
1.) Mix with adhesive, then coat: This sort of works, but results is a coating that is far from a monolayer; on hold for now.
2.) Sprinkle [great technical term] the starch onto an adhesive: Difficult to prevent tiny grains from clumping, hard to coat an even monolayer.
3.) Brush coating: The plate is coated with an adhesive, and then the starch is lightly brushed on. This works well, though care must be exercised not to put brush marks in the adhesive base. It is definetely doable, especially if pressure is applied to the plate after the coating is done.
4.) Coat plate wiht adhesive, submerge in liquid with suspension of starch: Basically, I mix the starch with water, place the plate in a container and pour the water / starch into it. The plate is then lifted, creating a coating of starch. This works ok, but has the issue of water remaining on the plate and disturbing hte adhesive / speading the starch around. Perhaps it would work with water containing a surfactant or some other liquid (acetone or ethanol, perhaps?)
For adhesive, i've experimented with everything from honey to epoxy. Epoxy seems to work well, though it hardens a little more quickly than I'd like.
An idea just occured to me. Ever seen those faux stained glass kits where you put the plastic stuff in the wire frame and heated it in the oven? If this kind of stuff could be gotten in the right size granules, mixed, and spread on glass in an even layer perhaps with some vibration to make it settle, then heated to make it bond to the glass. Result would be pretty much what you need wouldn't it?
Another thing may be to mix your starch with a dry powder that will fuse when heated to a temp low enough to not damage the starch. What that powder would be I'm not too sure of. Some kind of high temp wax maybe.
Another thing may be to mix your starch with a dry powder that will fuse when heated to a temp low enough to not damage the starch. What that powder would be I'm not too sure of. Some kind of high temp wax maybe.
I like that Idea. The main problem is that when melted, the stuff tends to bleed together, forming gooey gradients in plastic. However, I could simply grind the particles to a small size, and place them ina monolayer, with some moderate heating, or just spread some epoxy over them. That's an EXCELLENT idea.
I'll see if I can find one of those, though not sure where.
I'll see if I can find one of those, though not sure where.
err... what happened next...? 

I can't speak for Htmlguru, but I did make some (somewhat unexciting) progress this year. I haven't worked on anything for the last 6 months to focus on my Lippmann plates, but...
I bought some commercial potato starch and dyed it with standard food colorings. The result after mixing was a neutral gray powder. Actual autochrome starch in this form was much darker, so in the future I'll be mixing dye solutions to be much stronger.
I was able to make a pretty decent color screen. I used standard Elmer's rubber cement diluted with xylene 1:8, which formed a clear, runny, tacky varnish. There wasn't a large window between the varnish being too wet and being too dry to take on the starch, so coating was somewhat difficult. Maybe in the future I'll dilute with less xylene. I still have a few screens laying around if you want to see a picture, but they are somewhat unremarkable.
I was able to smash the starch with a tablespoon. This was an incredibly slow and tedious process, but it was the only way I was able to find without disturbing the starch (or breaking the plate!). I'm still brainstorming a better way to do this.
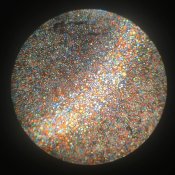
Standard graphite from the hardware store brushed over the starch fills the gaps pretty well. I wish my microscope had a camera, I would love to show off what the screen looks like under it! Fairly reminiscent of what you see on a search for "autochrome screen" on Google. The Lumieres used talc, I believe to remove excess graphite -- this didn't seem to work for me (I was using baby powder...) and just darkened the screen somewhat. Carefully brushing with a soft brush and blowing gently seemed to remove most of the excess graphite.
I used standard shellac varnish from the hardware store to seal the screen, but this lifted from the plate during processing. I realize the Lumieres used dammar gum in ethyl acetate for this step, so that's something I'll look into making in the future.
For red sensitizing the emulsion, I started with methyl violet as it was easily obtainable. I had no success with this at all whatsoever. Ethyl violet has been much more effective in my Lippmann plates, so I'll probably continue to use this down the road. I've been able to make standard orthochromatic emulsions up to about 20 ISO, though speed isn't a huge concern for me personally.
You know, writing this all out has got me a little excited to start playing with this stuff again. Hopefully I can get a little closer in 2016!
I bought some commercial potato starch and dyed it with standard food colorings. The result after mixing was a neutral gray powder. Actual autochrome starch in this form was much darker, so in the future I'll be mixing dye solutions to be much stronger.
I was able to make a pretty decent color screen. I used standard Elmer's rubber cement diluted with xylene 1:8, which formed a clear, runny, tacky varnish. There wasn't a large window between the varnish being too wet and being too dry to take on the starch, so coating was somewhat difficult. Maybe in the future I'll dilute with less xylene. I still have a few screens laying around if you want to see a picture, but they are somewhat unremarkable.
I was able to smash the starch with a tablespoon. This was an incredibly slow and tedious process, but it was the only way I was able to find without disturbing the starch (or breaking the plate!). I'm still brainstorming a better way to do this.
Standard graphite from the hardware store brushed over the starch fills the gaps pretty well. I wish my microscope had a camera, I would love to show off what the screen looks like under it! Fairly reminiscent of what you see on a search for "autochrome screen" on Google. The Lumieres used talc, I believe to remove excess graphite -- this didn't seem to work for me (I was using baby powder...) and just darkened the screen somewhat. Carefully brushing with a soft brush and blowing gently seemed to remove most of the excess graphite.
I used standard shellac varnish from the hardware store to seal the screen, but this lifted from the plate during processing. I realize the Lumieres used dammar gum in ethyl acetate for this step, so that's something I'll look into making in the future.
For red sensitizing the emulsion, I started with methyl violet as it was easily obtainable. I had no success with this at all whatsoever. Ethyl violet has been much more effective in my Lippmann plates, so I'll probably continue to use this down the road. I've been able to make standard orthochromatic emulsions up to about 20 ISO, though speed isn't a huge concern for me personally.
You know, writing this all out has got me a little excited to start playing with this stuff again. Hopefully I can get a little closer in 2016!
Red sensitization depends on emulsion some times.
What were you using?
PE
What were you using?
PE
Hey PE,
It was the Mark Osterman's emulsion. I didn't really have very good equipment though, so I was plagued with problems. I had run out of chrome alum without realizing it so my emulsion was lifting from the plates. It was about ISO 3 without any sensitizers.
At the time I was using a 1:100 methyl violet solution in denatured alcohol, added dropwise, and doubling the amount added to the emulsion after each trial. I started with 1 drop to 500mL of emulsion and went up until the plates were a deep purple (which interestingly enough, stopped the emulsion from lifting! At the expense of losing about 8 stops in speed...)
I'd be interested in trying this again at some point, now that I have proper equipment to make the stuff.
It was the Mark Osterman's emulsion. I didn't really have very good equipment though, so I was plagued with problems. I had run out of chrome alum without realizing it so my emulsion was lifting from the plates. It was about ISO 3 without any sensitizers.
At the time I was using a 1:100 methyl violet solution in denatured alcohol, added dropwise, and doubling the amount added to the emulsion after each trial. I started with 1 drop to 500mL of emulsion and went up until the plates were a deep purple (which interestingly enough, stopped the emulsion from lifting! At the expense of losing about 8 stops in speed...)
I'd be interested in trying this again at some point, now that I have proper equipment to make the stuff.
Well, any sensitizer is also a desensitizer.
You have to balance the two effects and that is just the beginning to learn about these things which many ignore.
Then there is "J" typing which takes place when the Iodide is added afterwards. It is based on location of the iodide.
Sensitizing dye amount is based on emulsion grain size, going down as size increases. You will have to determine this by trial and error.
PE
You have to balance the two effects and that is just the beginning to learn about these things which many ignore.
Then there is "J" typing which takes place when the Iodide is added afterwards. It is based on location of the iodide.
Sensitizing dye amount is based on emulsion grain size, going down as size increases. You will have to determine this by trial and error.
PE
Hey everyone, it's been a while.
I've been playing around with making these again for a few months. Here's a write up my progress so far. Hopefully some if you will find it interesting.
In summary:
1. Right now I've worked out most of the kinks as far as making the first varnish and dyeing/compressing the starch
2. I think I have a decent, working second varnish but I haven't been able to test it
3. Preliminary tests have failed to render color, due to fogged emulsion (inexperience on my part) and a second varnish layer that is too thick
4. I kept the same mg/mol dye ratios for erythrosine and pinacyanol as I do for my Lippmann plates. Now that I'm re-reading PE's last response from 2015, it's probably too much, due to larger grain size...
5. I've managed to source all the original sensitizing and starch dyes except for Orthochrome T. All my experiments up to here have been using easily available T-shirt dyes.
I'm hoping to get some color response in the next couple of week! I'll keep you posted...
I've been playing around with making these again for a few months. Here's a write up my progress so far. Hopefully some if you will find it interesting.
In summary:
1. Right now I've worked out most of the kinks as far as making the first varnish and dyeing/compressing the starch
2. I think I have a decent, working second varnish but I haven't been able to test it
3. Preliminary tests have failed to render color, due to fogged emulsion (inexperience on my part) and a second varnish layer that is too thick
4. I kept the same mg/mol dye ratios for erythrosine and pinacyanol as I do for my Lippmann plates. Now that I'm re-reading PE's last response from 2015, it's probably too much, due to larger grain size...
5. I've managed to source all the original sensitizing and starch dyes except for Orthochrome T. All my experiments up to here have been using easily available T-shirt dyes.
I'm hoping to get some color response in the next couple of week! I'll keep you posted...
Attachments
Remember, your dye grains must be larger than your emulsion grains. If they are smaller, then one grain can be exposed by 2 colors. However, if you invert the image to make a positive, as is proper for Autochromes, the trees are at least green. 
PE

PE
Yup! I think we're good in that department... I'm not sorting my starch grains right now, so they're magnitudes larger than the emulsion grains.Remember, your dye grains must be larger than your emulsion grains.
I attached a picture of a blue object (a trash can). It looks there might be some correlation there after all, though it looks like the exposed part of the emulsion are shifted down and to the left...
That's looking on the bright side!However, if you invert the image to make a positive, as is proper for Autochromes, the trees are at least green.
Attachments
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |