From the structures that dE fENDER has posted it is seems apparent that Ethyl Red Iodide is chemically very close to Othochrome T. Wall mentions that both dyes will sensitize to wave length 6200--aproximately orange in color.
That's good to know that I'm not entirely crazy. In the Lavendrine/Gandolfo book they state that Orthochrome T is used here as a green sensitizer, though all the information I could ever find about it indicated that it was more favorable as a red sensitizer. Strange that it was used in conjunction with ethyl violet in their sensitizing solution.
Here are a few new tests I have done:
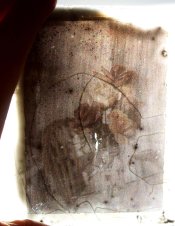
With the spin coated plate, I made the mistake of spinning the plate first, and adding the emulsion to the center. The middle of the plate is nice and even, but as you can see the emulsion liked either to "run away" off the side of the plate, or congealed thickly before it could get there. I think there is merit to this, but it might make more sense to coat the plate first and spin it to clear the excess emulsion. 40 minutes, subject ~EV 5-6
The 'pour off' plate, I had decided to coat and drain the plate again collodion style, and allowed it to sit levelly to dry. I decided to see what a monumental exposure would look like - the plate was exposed overnight for 10 hours. The result is still quite dark, though you can see a bit of a gradient of thickness (thicker in the top left, thinner in the bottom right). The black streaks are due to previous emulsion coatings -- it seems the edges of the emulsion dissolve the second varnish slightly and leave behind marks. My sensitizing solutions are alcoholic, which I bet is the culprit...
In IMG_9083, I decided to see what an exposure outside would look like. This was a 2m10s exposure, subject jumping between EV13-15 (clouds kept frustrating the exposure time). I was shooting for 1m30s in sun and had to compensate a bit. It's overexposed, maybe by a stop or so, but still seemingly quite dense.
So far with these positives, all seem to share the common traits of being generally very dark, while at the same time lacking the dynamic range of how they look when processed as negatives. 'spincoat1s', attached here, has the best transmittance here, and I feel 40 minutes for this scene is probably underexposed -- perhaps I'll revisit spin coating with a longer exposure. In my previous post, IMG_9073s seems to have the best dynamic range, despite being very dark.
I'm definitely open to continued suggestions for things to try... I'm starting to get stumped...