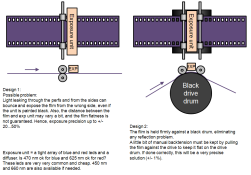
The pink elephant in the room, to my mind, is the coupler chemistry used. So far there's been no mention of it by Stephen, though much more curiously, no one has asked.
Is this something you'd be willing to share?
I've noticed many times in the past that Mssr. Frizza will achieve things that just blow people away here, and he's always quick to point out that none of it's magic; it's a result of actual work, understanding the process and simply doing it. I greatly respect this, and it's model behavior in an internet community that's all too content with typing thoughts instead of doing actions. (*guilty as charged*) That's probably why we don't see him so often either.
With that in mind I wouldn't expect to have the whole process divulged just for asking, but I do think that if there is no incentive to keep it "proprietary" (and perhaps there is) then the details might be useful to someone who actually wishes to give this a go at some point.
On the other hand, I can understand how sharing it might cheapen your hard work. Hell, at least Daguerre got a life-time pension for giving away his process...
-----------------------------------
p.s. On "(there was a url link here which no longer exists)", starting around post #3010 there is a discussion of color developers.
I havent gone into extensive detail simply because anything i write will pale in relation to the wealth of information already available online about every aspect of the kodachrome process. The extensive information on the chemistry, the processing and the film can be found in public domain by kodak. For interest this patent which is wonderfully half the work of Photo Engineer is here....
http://www.freepatentsonline.com/3658525.pdf
alternatively something more primitive you can try is....

If you want to know some info about the processing steps....
Processing Steps as per kodaks direction....
Backing Removal Solution
The alkaline backing removal solution converts the rem-jet
antihalation backing on the film base into a water-soluble
form. This backing is removed in the backing removal wash.
Backing Removal Wash
This wash performs two functions:
1. It removes the backing removal solution from the film.
2. It completely removes the solubilized antihalation
backing from the base by a combination of water
action and mechanical buffing.
First Developer Solution
In the first developer solution, the exposed silver halide
grains (latent images) are reduced to metallic silver by the
action of Phenidone* and hydroquinone developers:
The resulting silver grains form three superimposed
negative images of the original scene, one image in each of
the red-, green-, and blue-sensitive emulsion layers. The
remaining (unexposed and undeveloped) silver halide in the
three emulsion layers constitutes the positive (reversal)
images that are later converted to full-color images in the
color-development phases of the process.
First Developer Wash
This wash stops the development and removes the first
developer solution from the film.
Red Reexposure Printing Step
The red reexposure printing step completely exposes all of
the remaining silver halide in the red-sensitive (bottom)
emulsion layer so that the silver halide develops completely
in the cyan developer solution. At the same time, exposure
of any remaining silver halide in the blue- and greensensitive
layers must be avoided to prevent unwanted cyan
dye development in these layers. This selective exposure is
obtained by printing through the base side of the film, using
a properly selected red glass filter in the light beam. The
green- and blue-sensitive emulsion layers have no
intentional sensitivity to red light and should therefore
remain unaffected by the red-light exposure. However, some
green-sensitive emulsion layers do have a slight, but
significant, red sensitivity, and accurate control of the red
printing intensity is necessary.
Cyan Developer Solution
In the cyan developer solution, a positive silver image is
formed in the red-sensitive layer by the action of the color
developing agent on the silver halide that was exposed
during the red printing step Simultaneously, the resulting
oxidized color developer combines with the cyan coupler to
form a positive cyan dye image. This image is deposited
only in the red-sensitive emulsion layer.If any red-sensitive halide
is left undeveloped, unwanted dyes will be produced in the red-sensitive
layer during later.
Cyan Developer Wash
This wash stops cyan development and removes the cyan
developer solution from the film.
Blue Reexposure Printing Step
In this printing step, all the remaining silver halide in the
blue-sensitive top emulsion layer is exposed so that the silver
halide develops completely in the yellow developer solution.
At the same time, exposure of the remaining silver halide in
the green-sensitive layer (which is also blue-sensitive) must
be avoided to prevent unwanted yellow dye development in
the green-sensitive layer. This selective exposure is obtained
by printing through the emulsion surface of the film, using a
properly selected blue glass filter in the light beam. The
yellow filter layer between the blue- and green-sensitive
layers limits passage of blue light from the emulsion side.
However, the filter layer does not protect the green-sensitive
layer from any stray blue printing light that may strike the
base of the film.
An optimum printing intensity for each printer should be
established and then carefully controlled. Overprinting can
result in unwanted exposure and subsequent yellow
development of silver halide in the green-sensitive
(magenta) layer. Underprinting leaves some of the silver
halide in the yellow layer unexposed and subject to chemical
exposure and development in the magenta developer. Either
situation causes some degradation in quality.
The selected levels of reexposure for both the red and blue
printing steps are based on the results of actual photographic
tests including each of the film types that are processed.
These printer settings are computer controlled. For anything
other than a lamp failure, call Kodak for service. Processing
film with an inoperative printer produces unacceptable
customer film.
Yellow Developer Solution
In the yellow developer solution, a positive silver image is
formed in the blue-sensitive layer by the action of the color
developing agent on the silver halide that was exposed
during the blue printing operation. Simultaneously, a
positive yellow dye image is formed by the reaction between
the oxidized color developing agent and the yellow coupler.
See the section, Cyan Developer Solution on page 3-2 for
the generic equations.
During the yellow development step, the blue-sensitive
layer must be developed to completion while unwanted
yellow development (fogging) of the green-sensitive layer is
kept to a minimum. Any undeveloped silver halide in the
blue-sensitive layer is developed in the magenta developer
solution, causing magenta dye contamination in the yellow
layer. Conversely, fogging of the green-sensitive layer
during yellow development causes yellow dye
contamination in the magenta layer and a significant
reduction in the magenta dye yield. A normal Process
K-14M yellow developer solution provides the required
yellow and magenta separation.
Normally, all of the exposed silver halide in the redsensitive
layer would be developed in either the first or the
cyan developer solution. If any exposed silver halide in this
layer remains undeveloped after the cyan developer solution,
it is developed in the yellow developer solution, and results
in yellow dye contamination in the cyan layer.
Yellow Developer Wash
This wash stops the yellow development and removes the
yellow developer solution from the film.
Magenta Developer Solution
At this stage in the processing sequence, only the greensensitive
layer should contain any unexposed silver halide.
Therefore, selective reexposure is unnecessary. The reversal
agent in the magenta developer solution nucleates
(chemically reexposes) all the remaining silver halide.
During magenta development, a positive silver image is
formed in the green-sensitive layer by the action of the color
developing agent on the silver halide. Simultaneously, a
positive magenta dye image is formed by the reaction of the
oxidized color developing agent with the magenta coupler.
See the section, Cyan Developer Solution on page 3-2.
Magenta development is somewhat less critical than cyan
and yellow development, because if the preceding steps were
properly carried out, no silver halide should remain in the
red- and blue-sensitive layers. Therefore, no unwanted
magenta dye development should occur. However, if any
silver halide is present in the red- or blue-sensitive layers, it
is nucleated and developed in the magenta developer
solution, producing magenta dye contamination of the cyan
or yellow dye image.
The silver halide in the green-sensitive layer is the most
difficult to develop completely, and incomplete
development results in an inadequate magenta dye image,
especially in the maximum-density areas
Magenta Developer Wash
This wash removes the magenta developer solution from the
film. This is the most critical of all the wash steps because it
is more difficult to remove the components of the magenta
developer solution.
Conditioner
The conditioner prepares the metallic silver developed in the
first and color developers for oxidation to silver halide in the
bleach step. An oxidized conditioner solution is ineffective
and may cause silver to be retained in processed film.
Bleach
The bleach converts the metallic silver back to silver halide;
the silver halide is later removed in the fixer.
During bleaching, iron III is reduced to iron II. Iron II
must be converted back to iron III by aeration so that
satisfactory bleaching can continue. Aerate the bleach by
bubbling air through it.
Inadequate aeration, underreplenishment, low
temperature, and over-dilution of the bleach by conditioner
can cause silver retention, which causes all densities to
increase. The silver may be removed by bleaching and fixing
the film again, if necessary.
Fixer
The fixer converts all of the silver halide into soluble silver
compounds. Most of the silver compounds are removed in
the fixer and can be recovered.
Underreplenishment, or fixer dilution, causes silver halide
retention, increased blue density, or yellow D-min. The
silver halide may be removed by bleaching and fixing the
film again.
Final Wash
The final wash removes chemicals remaining in the film
emulsion. Complete washing at this stage is important for
image stability; any chemicals remaining in the film may
deteriorate the image dyes.
Final Rinse
The final rinse contains a wetting agent to reduce water
spotting and provide uniform drying. To help prevent water
spots and streaks, replace the final rinse solution daily
THATS A BASIC OVERIEW OF THE PROCESS AS DESCRIBED BY KODAK.
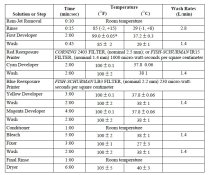
and here is a chart outlining the times and temps etc of each processing step...... (when hand processing certain times like the rem jet removal and wash time will need to be adjusted)
And any other detail under the sun about kodachrome from the way the film is made to the way a K-lab machine works can be found online in the Z-50 tech pubs, there is also the Klab user manuals etc...kodak have openly available everything and anything you want to know. I'm not going to re write the ins and outs of publications that in common knowledge already exist.