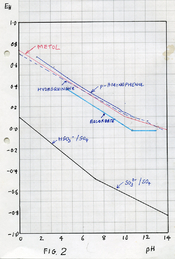
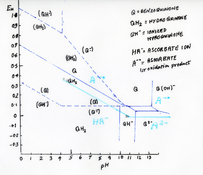
Yes, I do have a small stash of Catechol here. I am not a caffenol enthusiast, but assuming, that espresso drawn from a 6 servings Bialetti will do the job, I can do some quick experiments with photographic paper. One batch with roughly 30 g/l Potassium Carbonate (still have some stock solution sitting around here), some amount of coffee and either nothing (dev 1) or 5 g/l either Ascorbic Acid (dev 2) or Catechol (dev 3).
If Quercetin were indeed superadditive with Ascorbic Acid, then it should not be superadditive with Catechol, and there should be a profound difference between dev 2 vs. dev 1&3. If some other compound in coffee works as primary dev agent, then dev 2 and 3 should work more or less the same. Cross your fingers that I get dark room time on Wednesday.
I shall report here.
If Quercetin were indeed superadditive with Ascorbic Acid, then it should not be superadditive with Catechol, and there should be a profound difference between dev 2 vs. dev 1&3. If some other compound in coffee works as primary dev agent, then dev 2 and 3 should work more or less the same. Cross your fingers that I get dark room time on Wednesday.
I shall report here.