Does it need the high pH of Carbonate to get Quercetin into solution? This page claims that Quercetin's first pKa is 6.44, which tells me that much weaker alkalis (Borax, Sulfite) should dissolve it in quantity. There is a good chance you can make a stable stock solution at pH 7.5 that both contains Quercetin in higher amounts and is not overly prone to premature oxidation.
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Quercetin Chalcone develops like Pyrogallol
-
A
- Thread starter Alan Johnson
- Start date
Recent Classifieds
-
For Sale Kodak 2465 microfilm
- Started by MCB18
-
Want to Buy Linhof Technika 4x5 240 cam.
- Started by Logical1
-
Free Free Darkroom Stuff
- Started by Reinhold
-
For Sale Zeiss Distagon 35mm f2 ZF Industrial
- Started by Jammoh
-
For Sale FS: Fujinon 250mm f/6.7 Lens
- Started by B.S.Kumar
Forum statistics
- Joined
- Nov 16, 2004
- Messages
- 3,374
It is possible to make a borax solution but it is not very concentrated and develops more slowly than the carbonate version.
Metol.....................0.2g
Quercetin...............1.0g
Borax.....................3.0g
Water to...............100ml
In a stainless steel container, boil and simmer 5 min,filter through cotton wool (about half the Quercetin dissolves).
Make up to 100ml ,store in a full sealed bottle.Dilute 1+9 for use.
Development time is over 30 min.
Metol.....................0.2g
Quercetin...............1.0g
Borax.....................3.0g
Water to...............100ml
In a stainless steel container, boil and simmer 5 min,filter through cotton wool (about half the Quercetin dissolves).
Make up to 100ml ,store in a full sealed bottle.Dilute 1+9 for use.
Development time is over 30 min.
- Joined
- Nov 16, 2004
- Messages
- 3,374
A 1-shot carbonate version takes a long time to make but the Quercetin capsules,stored in their container , last indefinitely.
Suitable for occasional use of staining developer.
QS-1
Metol .............................0.1g
Quercetin........................0.5g
Sodium Carbonate anh......2.5g
Water to........................500ml
Weigh out solids into container, add 500ml just boiled hot water, stir, filter through cotton wool (the Quercetin does not all dissolve)
Develop 30min 20C.
Suitable for occasional use of staining developer.
QS-1
Metol .............................0.1g
Quercetin........................0.5g
Sodium Carbonate anh......2.5g
Water to........................500ml
Weigh out solids into container, add 500ml just boiled hot water, stir, filter through cotton wool (the Quercetin does not all dissolve)
Develop 30min 20C.
Attachments
- Joined
- Nov 16, 2004
- Messages
- 3,374
Quercetin alone without metol provides developers like weak caffenol.
Qu-2
Water(room temp)...........................500 ml
Washing soda,Arm & Hammer (US).....4.5 tsp
or washing soda decahydrate (UK).......10 tsp
Iodized salt......................................0.5 tsp
Quercetin, 2 capsules contents,..........1.0 g
Stir 15 min,filter through cotton wool.
I semi-stand developed APX 100 in this 2hrs 70F, agitate every 30 min.
The negatives are much fogged but strongly stained and tanned.
Qu-3
As Qu-2 with addition of 1 tsp vitamin C.
I developed APX 100 in this 30 min 68F, agitate 10s/minute.
The negatives are somewhat fogged but produce sharp ,fairly fine grained prints.
There is only weak tanning, probably not enough to control highlight density.
Likely the reason why addition of vitamin C speeds up the development is that in Qu-3 it removes the oxidation products found with Qu-2 that tan the gelatin.
Qu-2
Water(room temp)...........................500 ml
Washing soda,Arm & Hammer (US).....4.5 tsp
or washing soda decahydrate (UK).......10 tsp
Iodized salt......................................0.5 tsp
Quercetin, 2 capsules contents,..........1.0 g
Stir 15 min,filter through cotton wool.
I semi-stand developed APX 100 in this 2hrs 70F, agitate every 30 min.
The negatives are much fogged but strongly stained and tanned.
Qu-3
As Qu-2 with addition of 1 tsp vitamin C.
I developed APX 100 in this 30 min 68F, agitate 10s/minute.
The negatives are somewhat fogged but produce sharp ,fairly fine grained prints.
There is only weak tanning, probably not enough to control highlight density.
Likely the reason why addition of vitamin C speeds up the development is that in Qu-3 it removes the oxidation products found with Qu-2 that tan the gelatin.
Hi all, going back to film after giving up on digital as a legitimate art form. As I update myself on films and chemistries, I am equally saddened by the end of Verichrome Pan and gladdened by the advent of films like Acros.
But "Holy-If-It-Works-Don't-Fix-It-Batman!! Sorting out the innumerable Super New Magic Ju-Ju Water/TEA/PG Brews will drive a guy nuts. (Short trip.) Back in the '70s, I thought adding a bit of benzotriazole to D-23 was advanced darkroom chemistry, but now--
FWIW, I'm thinking Acros in either GSD-10 (simple and I always liked Ansco130 for prints; ahhh glycin) or Halcyon (has that Harvey's Defender 777 mystique) will be my first choices, having come to pray at the Oracle d'Fehr.
Enjoy reading all this stuff, but being old-school, I expect my film chemistry to be toxic. Making developer out of flavinoids and coffee just feels wrong.
Now where'd I put that cigar..? :munch:
But "Holy-If-It-Works-Don't-Fix-It-Batman!! Sorting out the innumerable Super New Magic Ju-Ju Water/TEA/PG Brews will drive a guy nuts. (Short trip.) Back in the '70s, I thought adding a bit of benzotriazole to D-23 was advanced darkroom chemistry, but now--
FWIW, I'm thinking Acros in either GSD-10 (simple and I always liked Ansco130 for prints; ahhh glycin) or Halcyon (has that Harvey's Defender 777 mystique) will be my first choices, having come to pray at the Oracle d'Fehr.
Enjoy reading all this stuff, but being old-school, I expect my film chemistry to be toxic. Making developer out of flavinoids and coffee just feels wrong.
Now where'd I put that cigar..? :munch:
relistan
Member
Quercetin alone without metol provides developers like weak caffenol.
Qu-2
Water(room temp)...........................500 ml
Washing soda,Arm & Hammer (US).....4.5 tsp
or washing soda decahydrate (UK).......10 tsp
Iodized salt......................................0.5 tsp
Quercetin, 2 capsules contents,..........1.0 g
Stir 15 min,filter through cotton wool.
I semi-stand developed APX 100 in this 2hrs 70F, agitate every 30 min.
The negatives are much fogged but strongly stained and tanned.
Qu-3
As Qu-2 with addition of 1 tsp vitamin C.
I developed APX 100 in this 30 min 68F, agitate 10s/minute.
The negatives are somewhat fogged but produce sharp ,fairly fine grained prints.
There is only weak tanning, probably not enough to control highlight density.
Likely the reason why addition of vitamin C speeds up the development is that in Qu-3 it removes the oxidation products found with Qu-2 that tan the gelatin.
Alan, I was reading Haist again and decided to try to figure out what I could about which ingredients are active in caffenol. I found an article measuring the amounts of phenols and flavonoids in coffee https://link.springer.com/article/10.1007/s00217-019-03388-9 . I was going through the list and looking at the molecular structures and came across Quercetin. When I googled this to see if anyone had used it directly as a developing agent, I of course found your thread.
I see that the price on Quercetin has gone down a lot and might be worth playing with again. https://oxfordvitality.co.uk/products/quercetin-powder?variant=34728403828902
It sounds like solubility is the main issue. Did you figure out a nicer way to get it to fully dissolve? Seems like higher concentration is necessary to make something really useful.
relistan
Member
Furthermore I forgot to post that this article https://pubs.rsc.org/en/content/articlelanding/1996/p2/p29960002497 says:
According to Haist, one measure of whether or not an agent will be good for development (aside from the other characteristics which Quercetin has) is if it can overcome the initial inertia required to start development. This requires a negative potential of something like 80 mV to be effective. D-76 with the full formula has a negative potential of 259 mV. D-72 has 407 mV. Too high will result in developing unexposed silver, so there is a Goldilocks region where the potential needs to be.
Quercetin, if I understand that article, is something like 330 mV. That theoretically puts Quercetin right in the range of being very effective on its own.
Quercetin is the best electron donor of all investigated flavonoids (measured E10.8= 0.09 V, and calculated E7= 0.33 V). The favourable electron-donating properties originate from the electron donating 0-3 hydroxy group in the C ring, which is conjugated to the catechol (B ring) radical through the 2,3-double bond
According to Haist, one measure of whether or not an agent will be good for development (aside from the other characteristics which Quercetin has) is if it can overcome the initial inertia required to start development. This requires a negative potential of something like 80 mV to be effective. D-76 with the full formula has a negative potential of 259 mV. D-72 has 407 mV. Too high will result in developing unexposed silver, so there is a Goldilocks region where the potential needs to be.
Quercetin, if I understand that article, is something like 330 mV. That theoretically puts Quercetin right in the range of being very effective on its own.
In neutral and slightly alkaline media (pH 7–9), all investigated flavonoids are inferior electron donors to ascorbate. Quercetin, E7= 0.33 V, and gallocatechins, E7= 0.43 V, can reduce vitamin E radicals (assuming the same reduction potential as Trolox C radicals, E7= 0.48 V).
Last edited:
- Joined
- Nov 16, 2004
- Messages
- 3,374
Furthermore I forgot to post that this article https://pubs.rsc.org/en/content/articlelanding/1996/p2/p29960002497 says:
According to Haist, one measure of whether or not an agent will be good for development (aside from the other characteristics which Quercetin has) is if it can overcome the initial inertia required to start development. This requires a negative potential of something like 80 mV to be effective. D-76 with the full formula has a negative potential of 259 mV. D-72 has 407 mV. Too high will result in developing unexposed silver, so there is a Goldilocks region where the potential needs to be.
Quercetin, if I understand that article, is something like 330 mV. That theoretically puts Quercetin right in the range of being very effective on its own.
Interesting abstract Karl.
From my result post 30, Quercetin alone is not very active ,Qu-2 but is boosted by ascorbate, Qu-3.
This suggests that ascorbate reacts with the oxidation products of Quercetin which are adsorbed on the grains.
But still the development is slow. It seems to be adsorption of oxidation products that makes Quercetin less active than the Haist article and the abstract suggest.
This conclusion is supported by the results of Jay DeFehr who found that Quercetin provided a low contrast microfilm developer in one of his experiments [Oct 9]:

Superfine grain film developer
Qu-1, Quercetin-metol-carbonate was also pretty slow. In this case the quercetin is very slow to regenerate the metol oxidation products, if in fact it actually does anything.
So I agree with your conclusion that the best way to speed things up might be to increase the quantity of quercetin dissolved.
I never figured out a better way to get quercetin to dissolve, maybe it might dissolve in isopropanol.
I will eventually reply to the link in post 32 in the following thread about coffee developer:

Caffenol active substances
I’ve been experimenting with Caffenol for a while, mostly using Fompan 400, and gotten pretty good results. I’n curious about what ingredient does what? Say I wanted to make the solution stronger, would I add more of everything, just more coffee? More soda? Merry Christmas everyone!
Last edited:
Reduction potential is just one factor, a necessary one but by itself not sufficient. Some capable reducers (e.g. Ascorbic Acid) are very effective together with a primary development agent, but are very weak developers on their own. There are strong reducers with no development activity at all (e.g. Sodium Sulfite). These 330mV are not a constant either: pH certainly has an effect, and most likely also its concentration.Quercetin, if I understand that article, is something like 330 mV. That theoretically puts Quercetin right in the range of being very effective on its own.
It's complicated ...
relistan
Member
Reduction potential is just one factor, a necessary one but by itself not sufficient. Some capable reducers (e.g. Ascorbic Acid) are very effective together with a primary development agent, but are very weak developers on their own. There are strong reducers with no development activity at all (e.g. Sodium Sulfite). These 330mV are not a constant either: pH certainly has an effect, and most likely also its concentration.
It's complicated ...
Yeah thanks Rudi. I was focusing on reduction potential because we already knew it was capable of operating as a developing agent and has a ring that is a catechol. The link says that the reduction potential of 330 mV is between pH 7-9, meaning the sweet spot of 8-9 is covered. I was trying to suggest that given the right circumstances, it looks potentially promising as a primary agent.
But getting it into solution in water is the challenge. And I agree your last point about concentration being a likely angle.
Last edited:
This is a lot of work. I had to look up 3 of the 5 words in the thread title just to read anything. But now I know that "Quercetin is a flavonoid widely distributed in nature. The name has been used since 1857, and is derived from quercetum (oak forest), after Quercus"
I'll sleep better tonight knowing that.
I'll sleep better tonight knowing that.
Yeah thanks Rudi. I was focusing on reduction potential because we already knew it was capable of operating as a developing agent and has a ring that is a catechol. The link says that the reduction potential of 330 mV is between pH 7-9, meaning the sweet spot of 8-9 is covered. I was trying to suggest that given the right circumstances, it looks potentially promising as a primary agent.
Sorry for being a nitpick, but the article you quoted states, that this reduction potential of 330mV is the value for pH 7. As with most developers, there is a strong relationship between pH and reduction potential (see the public version of "Theory of the Photographic Process", pages 478ff). This number puts Quercetin right into the group of Catechol, Hydroquione and the likes.
relistan
Member
Sorry for being a nitpick, but the article you quoted states, that this reduction potential of 330mV is the value for pH 7. As with most developers, there is a strong relationship between pH and reduction potential (see the public version of "Theory of the Photographic Process", pages 478ff). This number puts Quercetin right into the group of Catechol, Hydroquione and the likes.
I see, I was misreading how the numbers worked from the statement about ph 7-9. Well, that's still of interest as a secondary agent. I didn't expect it to exceed catechol, but I don't have numbers for that that I can find so far.
- Joined
- Nov 16, 2004
- Messages
- 3,374
relistan
Member
HQ vs pH is here:
Very helpful, thanks!
I see, I was misreading how the numbers worked from the statement about ph 7-9. Well, that's still of interest as a secondary agent. I didn't expect it to exceed catechol, but I don't have numbers for that that I can find so far.
It may work as primary or secondary development agent. If it is just a secondary development agent, then the search for the primary development agent in coffee continues. Something activates the ascorbate, which is an exceedingly poor developer on its own.
BTW don't get hung up on its precise reduction potential. Some adjustment of pH alleviates all these differences between developers.
relistan
Member
It may work as primary or secondary development agent. If it is just a secondary development agent, then the search for the primary development agent in coffee continues. Something activates the ascorbate, which is an exceedingly poor developer on its own.
BTW don't get hung up on its precise reduction potential. Some adjustment of pH alleviates all these differences between developers.
I think the likely outcome is that there are several “primary” agents in caffenol, all appearing to be forms of catechol. But it would be nice if a more deterministic outcome were possible by isolating one or more of the agents. I think Alan has shown that the right formula of a Quercetin developer might be a starting point. That was my interest.
relistan
Member
Mees’ book is the one @Rudeofus was referring to earlier. I did not know there was a public version available. It is here https://archive.org/details/TheTheoryOfThePhotographicProcess/page/n477/mode/1up
I think the likely outcome is that there are several “primary” agents in caffenol, all appearing to be forms of catechol. But it would be nice if a more deterministic outcome were possible by isolating one or more of the agents. I think Alan has shown that the right formula of a Quercetin developer might be a starting point. That was my interest.
Catechol is not really known as primary developer, which would be superadditive with ascorbate. There have to be some other compounds in coffee, which serve this purpose.
relistan
Member
Catechol is not really known as primary developer, which would be superadditive with ascorbate. There have to be some other compounds in coffee, which serve this purpose.
I don’t know how specifically relevant it is but I had read here https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4444905/ that ascorbic acid in enough concentration can revert the oxidation product of catechol. Whether the products were dealing with here is a similar quinone, I don’t know. But it says
“Ascorbic acid could reduce the formed quinone instantly to the original substrate (catechol) at high concentration (>1.5 %) while at lower concentrations acted as competitive inhibitor (KI = 0.256 ± 0.067 mM).”
Caffenol-C-M has 16g of ascorbic acid in 1L of solution.
Maybe I’m still barking up the wrong tree but having looked at all the stuff known in coffee from the available papers, it really looks like it’s one or more of the molecules with a catechol attached.
relistan
Member
Actually, I think what I said above is in line with what @Alan Johnson concluded in #30.
- Joined
- Nov 16, 2004
- Messages
- 3,374
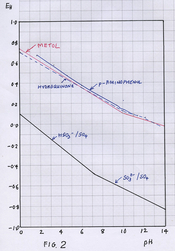
The oxidation/reduction potentials of ascorbate are well below those of HQ on the diagram post 40 [I can add them later].
If the various food derivatives are similar in oxidation/reduction potential to HQ, as was suggested for quercetin above, then in theory they could be regenerated by ascorbate.
Its not clear, IMO, if what is to be regenerated is the free unstable first oxidation product of the food derivative [this is called a semiquinone] or an oxidation product of the food derivative adsorbed on the surface of the grains, or a mixture of the two.
Anyhow it looks like its usually the ascorbate that does the regenerating.
If the various food derivatives are similar in oxidation/reduction potential to HQ, as was suggested for quercetin above, then in theory they could be regenerated by ascorbate.
Its not clear, IMO, if what is to be regenerated is the free unstable first oxidation product of the food derivative [this is called a semiquinone] or an oxidation product of the food derivative adsorbed on the surface of the grains, or a mixture of the two.
Anyhow it looks like its usually the ascorbate that does the regenerating.
Based on redox potential sulfite could reduce oxidized Phenidone as well, but it doesn't. It could reduce silver ions directly, but it doesn't. It could reduce Quinone back to Hydroquinone, but instead forms a sulfonate. From reading through investigated reactions of quinones in food stuff, most seem to happen in acidic environment, not where our developers typically operate.
It would be interesting, whether caffenol would also work, if we replaced the ascorbate with Catechol. This should answer in one set, whether the catechol derivatives in caffenol are reduced by the ascorbates, or whether some primary dev agent is hidden in coffee, which unleashes the ascorbate's development action.
It would be interesting, whether caffenol would also work, if we replaced the ascorbate with Catechol. This should answer in one set, whether the catechol derivatives in caffenol are reduced by the ascorbates, or whether some primary dev agent is hidden in coffee, which unleashes the ascorbate's development action.
relistan
Member
Do you have any catechol? I don't but this would be interesting.Based on redox potential sulfite could reduce oxidized Phenidone as well, but it doesn't. It could reduce silver ions directly, but it doesn't. It could reduce Quinone back to Hydroquinone, but instead forms a sulfonate. From reading through investigated reactions of quinones in food stuff, most seem to happen in acidic environment, not where our developers typically operate.
It would be interesting, whether caffenol would also work, if we replaced the ascorbate with Catechol. This should answer in one set, whether the catechol derivatives in caffenol are reduced by the ascorbates, or whether some primary dev agent is hidden in coffee, which unleashes the ascorbate's development action.
I am in the process of getting a pouch of quercetin powder, but it probably won't arrive for a couple of weeks. Then I can do a little experimenting to see if we can get more if it into solution. A heavily alkaline solution seems to be the best route, but I can't find any information about whether or not it is soluble in triethanolamine. I think this is because most work with it is for human consumption and thus it's not studied. When I manage to get the quercetin, I would like to first try @Alan Johnson's formula above but with 16g of ascorbic acid. Then do some experiments with solubility.
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |