@Jerry: The symptoms of crystallization are the developer turning white like milk, and then separating over days. This happened to me once when there was no developer present. But the concentration was extremely high because I was trying to find a solubility-limit for PG. So I learned that PG + sodium metaborate + ascorbic acid can crystallize. The Dimezone S seems to encourage and/or cause it though. So I think something other than hydrolysis is happening.
Also, Phenidone's higher fog is based only on my observations. But when I drop pH, fog-levels are comparable. One interesting thing I've noticed: Phenidone is almost exactly twice as active as Dimezone S in these formulations I've been testing. Odd, isn't it? Formulas I've seen more commonly use a factor of something like 1.33. But my densitometer tells me it's 2.0. Go figure...
@Alan: Regarding the 3-5 year lifetime of Mytol: Was your Mytol the original created by Paul Lewis, or the organic concentrate created by Jordan Wosnick which he calls "Instant Mytol"? I have a bottle of the concentrate mixed 9 months ago, and it shows no sign of crystallization.
Anyway, this crystallization-issue with Dimezone-S got me thinking about a developer I devised 6 months ago using Phenidone. So I re-mixed and ran another test-strip with it. Here's the one-litre formula for mixing directly into water:
45 g Sodium sulfite
2 g Sodium metaborate
4.5 g Ascorbic acid
2.45 g Propylene glycol (yes, grams)
0.05 g Phenidone (which was premixed into the above PG as a 2% solution)
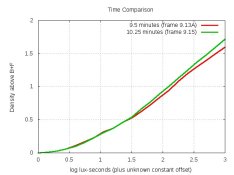
pH = 8.05 to 8.1. For TMY2: 13.25 minutes at 20C.
And the density-curves comparing it with XTOL:
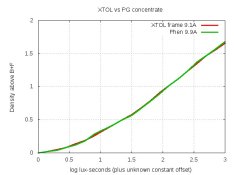
I've never seen such close graphs. The grain looks the same as XTOL under close exam on the light-table with 22X loupes.
Keeping sulfite separate, this formula should be able to be dissolved into PG, with a total volume of 20 ml, yielding a concentration ratio of 6.55/20 = 0.33, and a dilution of 1+49.
Alan: This is the same as D-316, but with the metaborate changed from 2.2 g to 2.0 g. So it's almost the same developer.
But it's rather different from XTOL. The dev-times are different, and more importantly, films respond to pH differently, so dev-times won't change by a simple factor. So this would require more testing than an XTOL-clone.
I think I'll try an XTOL-clone with Dimezone-S again, but using TEA instead of sodium metaborate. If the crystallization is from by-products of the reaction of metaborate with ascorbic acid, then TEA might fix the problem.
Mark Overton



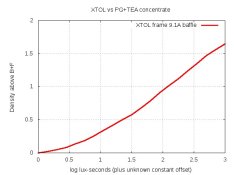
 That means we cannot allow this crystallization to occur. Any ideas how?
That means we cannot allow this crystallization to occur. Any ideas how?