The choice of sequestering agents that can be used in an ascorbate developer is very limited. EDTA, for example, will actually promote the oxidation of the ascorbate ion. This has been menthioned many times and by Ryuji himself.
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Progress on XTOL-concentrate
-
A
- Thread starter albada
- Start date
Recent Classifieds
-
For Sale Componon-S 5,6/300 and Componon-S 6,8/360 Enlarging lenses
- Started by alanbradford
-
For Sale Leica, Weston meters and Nikon filters.
- Started by Logical1
-
Want to Buy Fujinon A180mm F/9
- Started by loccdor
-
For Sale FS: Linhof 18x24cm Plate Holders
- Started by B.S.Kumar
-
Under Offer Several Pentax K-mount camera bodies
- Started by madsox
Forum statistics
The choice of sequestering agents that can be used in an ascorbate developer is very limited. EDTA, for example, will actually promote the oxidation of the ascorbate ion. This has been menthioned many times and by Ryuji himself.
Oxidation of ascorbate should be a non issue if you mix fresh from concentrate and use right away. I am aware of Ryuji's statements but unlike Mark's recipe Ryuji's published recipes are not concentrates in PG. The sequestrant I suggested should at least take care of Ca2+ and Mg2+, therefore I suggested Na2-EDTA and Calgon.
Just a note: if you source all the chemicals from Photographer's Formulary, each batch costs $3.71 without shipping. The most expensive ingredient is $1.03 of Propylene Glycol. This is assuming you only buy quantities <$10, except the Sodium Sulfite which is $17 for 5 lbs.
Acros-100 results
Two rolls of Acros-100 developed in XTOL and the concentrate look identical. The details are below. My testing so far shows that the concentrate behaves like XTOL for Kodak, Ilford and Fuji emulsions; for T-grain and traditional grain; and for 100- and 400-speed.
The concentrate had been kept in the refrigerator for a week, in case anyone cares. Here's a graph from a Stouffer wedge on my light-table, showing the density-curves for XTOL and the concentrate:
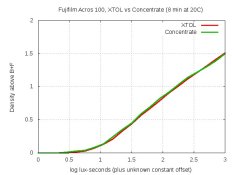
And here are full-resolution (2900 dpi) crops of neg-scans of the same frame-numbers from each of the two rolls:
Frame 24: Xtol: Concen:
Concen: 
Frame 25: Xtol: Concen:
Concen: 
Frame 26: Xtol: Concen:
Concen: 
The curves, grain and sharpness are all essentially identical to my eye.
@Rudeofus: Ryuji said that APX-100 and Pan-F worked poorly in DS-10. Pan-F is still in production, so I'll order some rolls and try it. Thanks for posting that idea. BTW, I thought Ryuji speculated that the failures were caused by DS-10's low pH of 8.0. I'm running at 8.3, which is a tad above XTOL and the same as D-76, so at least the concentrate won't have problems due to low pH. Nonetheless, I'm going to test Pan-F.
What problems do dissolved minerals in water cause? A search of apug.org shows some calcium specks on negatives if it precipitated out of solution. Also, folks say that carbonate causes calcium to precipitate, which my concentrate lacks, so hopefully that won't be a problem. My tap water is hard, so I'll try it on test-strips tomorrow.
One problem with sequestrants is that many are inorganic salts, and thus won't dissolve in PG. Citric acid is an exception, but it's a rather strong acid that I'd need to compensate for somehow.
@choppastyle: From a cost point of view, a low-volume shooter will save a little money using the concentrate. But my main motivations are (1) I hate to discard litres of expired developer every 6 months, (2) after 4 months, I lose confidence in XTOL and wonder if sudden death will hit me, and the concentrate will give me confidence for much longer, and (3) a little sulfite precipitates out of Xtol's solution, which looks like shredded tissue floating in it, and a poster said those are "hot" and can cause overdeveloped spots on negs. I've had that shredded tissue issue in both of my batches of Xtol, and solved it by vigorously shaking the bottle when warmed. The concentrate won't have that problem as it's mixed shortly before use.
I'll do more tests, but the big test now will be longevity, and that takes time.
Mark Overton
EDIT: For the Acros rolls: Xtol leader density was 3.09, and concentrate leader was 3.10.
Two rolls of Acros-100 developed in XTOL and the concentrate look identical. The details are below. My testing so far shows that the concentrate behaves like XTOL for Kodak, Ilford and Fuji emulsions; for T-grain and traditional grain; and for 100- and 400-speed.
The concentrate had been kept in the refrigerator for a week, in case anyone cares. Here's a graph from a Stouffer wedge on my light-table, showing the density-curves for XTOL and the concentrate:

And here are full-resolution (2900 dpi) crops of neg-scans of the same frame-numbers from each of the two rolls:
Frame 24: Xtol:
 Concen:
Concen: 
Frame 25: Xtol:
 Concen:
Concen: 
Frame 26: Xtol:
 Concen:
Concen: 
The curves, grain and sharpness are all essentially identical to my eye.
@Rudeofus: Ryuji said that APX-100 and Pan-F worked poorly in DS-10. Pan-F is still in production, so I'll order some rolls and try it. Thanks for posting that idea. BTW, I thought Ryuji speculated that the failures were caused by DS-10's low pH of 8.0. I'm running at 8.3, which is a tad above XTOL and the same as D-76, so at least the concentrate won't have problems due to low pH. Nonetheless, I'm going to test Pan-F.
What problems do dissolved minerals in water cause? A search of apug.org shows some calcium specks on negatives if it precipitated out of solution. Also, folks say that carbonate causes calcium to precipitate, which my concentrate lacks, so hopefully that won't be a problem. My tap water is hard, so I'll try it on test-strips tomorrow.
One problem with sequestrants is that many are inorganic salts, and thus won't dissolve in PG. Citric acid is an exception, but it's a rather strong acid that I'd need to compensate for somehow.
@choppastyle: From a cost point of view, a low-volume shooter will save a little money using the concentrate. But my main motivations are (1) I hate to discard litres of expired developer every 6 months, (2) after 4 months, I lose confidence in XTOL and wonder if sudden death will hit me, and the concentrate will give me confidence for much longer, and (3) a little sulfite precipitates out of Xtol's solution, which looks like shredded tissue floating in it, and a poster said those are "hot" and can cause overdeveloped spots on negs. I've had that shredded tissue issue in both of my batches of Xtol, and solved it by vigorously shaking the bottle when warmed. The concentrate won't have that problem as it's mixed shortly before use.
I'll do more tests, but the big test now will be longevity, and that takes time.
Mark Overton
EDIT: For the Acros rolls: Xtol leader density was 3.09, and concentrate leader was 3.10.
Last edited by a moderator:
Very nice!The curves, grain and sharpness are all essentially identical to my eye.
Let's not forget: DS-10 also contains a mild silver solvent, TEA. I read somewhere that this, together with long dev times, is the main culprit, why DS-10 doesn't do well with ultra fine grained film stock.Ryuji said that APX-100 and Pan-F worked poorly in DS-10. Pan-F is still in production, so I'll order some rolls and try it. Thanks for posting that idea. BTW, I thought Ryuji speculated that the failures were caused by DS-10's low pH of 8.0. I'm running at 8.3, which is a tad above XTOL and the same as D-76, so at least the concentrate won't have problems due to low pH.
I'd be afraid of calcium precipitation in any alkaline environment, carbonate or not. There must be a reason why so many recipes contain Calgon and/or EDTA.What problems do dissolved minerals in water cause? A search of apug.org shows some calcium specks on negatives if it precipitated out of solution. Also, folks say that carbonate causes calcium to precipitate, which my concentrate lacks, so hopefully that won't be a problem. My tap water is hard, so I'll try it on test-strips tomorrow.
PS: If EDTA and Calgon don't want to dissolve in PG under any circumstances, you could always add them to your sulfite component.
The results look very nice Mark. I'm impressed.
BTW, that must have been a windy day! Try looking at the images rapidly and you will see what I mean.
PE
BTW, that must have been a windy day! Try looking at the images rapidly and you will see what I mean.
PE
So, could you reprint the formula with the order of mixing?
Thanks
Thanks
Stress-test with 1+1 dilution
I wanted to test the capacity of this concentrate compared to XTOL. Kodak recommends at least 100 ml of XTOL be used per roll. So I used 100 ml, with 1+1 dilution (200 ml total), for 9 min at 20C (per the MDC). And same with the concentrate. Density-graph and scan-crops are below for Tri-X. The graph is interesting: It shows that under this capacity-stressing condition, the concentrate performed a hair better than XTOL, in that the densities of midtones and highlights are slightly higher. But so slight that folks would probably not notice the difference. Grain and sharpness look the same to me.
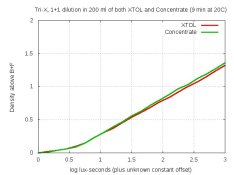
Consistent with the graph, the leaders were 2.03 for XTOL, and 2.07 for the concentrate.
Frame 20: Xtol: Concen:
Concen: 
Frame 21: Xtol: Concen:
Concen: 
Frame 22: Xtol: Concen:
Concen: 
This test also provides a second comparison of emulsions with conventional grains (ie, not T-grain).
A question for PE: The low densities in the graph and leaders imply underdevelopment, yet the short toe implies normal development. Can you explain this paradox?
Mark Overton
I wanted to test the capacity of this concentrate compared to XTOL. Kodak recommends at least 100 ml of XTOL be used per roll. So I used 100 ml, with 1+1 dilution (200 ml total), for 9 min at 20C (per the MDC). And same with the concentrate. Density-graph and scan-crops are below for Tri-X. The graph is interesting: It shows that under this capacity-stressing condition, the concentrate performed a hair better than XTOL, in that the densities of midtones and highlights are slightly higher. But so slight that folks would probably not notice the difference. Grain and sharpness look the same to me.

Consistent with the graph, the leaders were 2.03 for XTOL, and 2.07 for the concentrate.
Frame 20: Xtol:
 Concen:
Concen: 
Frame 21: Xtol:
 Concen:
Concen: 
Frame 22: Xtol:
 Concen:
Concen: 
This test also provides a second comparison of emulsions with conventional grains (ie, not T-grain).
A question for PE: The low densities in the graph and leaders imply underdevelopment, yet the short toe implies normal development. Can you explain this paradox?
Mark Overton
My replies to postings:
@Rudeofus: It has recently (2010) been discovered that metaborate sequesters cations such as Ca++. Search for "metaborate sequester". Here's a sentence from what appears to be the first report about this: "A form of borax known as metaborate has been found to sequester divalent cations such as Ca++ and prevent precipitation." The concentrate has a moderate amount of metaborate in it, so perhaps it can keep the calcium in check. How do I test sequestering of calcium and magnesium? I hope to run a test-strip with my hard tap-water this evening, but if it works (no calcium spots), I'm not sure that means anything.
@PE: There are no mountains between me and the Pacific ocean, so we get an ocean breeze in the afternoon, moderating the summer heat. Pleasant. We also get wildfires and earthquakes. Unpleasant.
We also get wildfires and earthquakes. Unpleasant. 
@mikebarger: Here's the formula:
Propylene glycol ............ 24 ml
DimezoneS/Phenidone ......... 0.2 / 0.105 g (DimezoneS dissolves in 3-5 minutes)
Sodium metaborate 4 mol ..... 6.7 g (dissolves in 3-5 minutes; turns orange)
Ascorbic acid ............... 8.5 g (dissolves in 7-10 minutes; fizzes and turns clear)
Propylene glycol to ......... 33.3 ml (final volume; should need to add little)
Heat to 90C to dissolve everything and drive the water out of the metaborate.
Dissolve in the order shown, and dissolve completely before adding the next chemical.
To make one litre of developer, mix 33.3 ml of concentrate into water containing 90 grams of sodium sulfite. That's 1+29 dilution.
The specific gravity is 1.18, so 33.3 ml of concentrate weighs 39.3 g, letting you measure it by weight if desired.
Times are same as XTOL. Target pH is 8.33.
Mark Overton
@Rudeofus: It has recently (2010) been discovered that metaborate sequesters cations such as Ca++. Search for "metaborate sequester". Here's a sentence from what appears to be the first report about this: "A form of borax known as metaborate has been found to sequester divalent cations such as Ca++ and prevent precipitation." The concentrate has a moderate amount of metaborate in it, so perhaps it can keep the calcium in check. How do I test sequestering of calcium and magnesium? I hope to run a test-strip with my hard tap-water this evening, but if it works (no calcium spots), I'm not sure that means anything.
@PE: There are no mountains between me and the Pacific ocean, so we get an ocean breeze in the afternoon, moderating the summer heat. Pleasant.
 We also get wildfires and earthquakes. Unpleasant.
We also get wildfires and earthquakes. Unpleasant. 
@mikebarger: Here's the formula:
Propylene glycol ............ 24 ml
DimezoneS/Phenidone ......... 0.2 / 0.105 g (DimezoneS dissolves in 3-5 minutes)
Sodium metaborate 4 mol ..... 6.7 g (dissolves in 3-5 minutes; turns orange)
Ascorbic acid ............... 8.5 g (dissolves in 7-10 minutes; fizzes and turns clear)
Propylene glycol to ......... 33.3 ml (final volume; should need to add little)
Heat to 90C to dissolve everything and drive the water out of the metaborate.
Dissolve in the order shown, and dissolve completely before adding the next chemical.
To make one litre of developer, mix 33.3 ml of concentrate into water containing 90 grams of sodium sulfite. That's 1+29 dilution.
The specific gravity is 1.18, so 33.3 ml of concentrate weighs 39.3 g, letting you measure it by weight if desired.
Times are same as XTOL. Target pH is 8.33.
Mark Overton
- removed account8
- Deleted
Thanks
My replies to postings:
@Rudeofus: It has recently (2010) been discovered that metaborate sequesters cations such as Ca++. Search for "metaborate sequester". Here's a sentence from what appears to be the first report about this: "A form of borax known as metaborate has been found to sequester divalent cations such as Ca++ and prevent precipitation." The concentrate has a moderate amount of metaborate in it, so perhaps it can keep the calcium in check. How do I test sequestering of calcium and magnesium? I hope to run a test-strip with my hard tap-water this evening, but if it works (no calcium spots), I'm not sure that means anything.
@PE: There are no mountains between me and the Pacific ocean, so we get an ocean breeze in the afternoon, moderating the summer heat. Pleasant.We also get wildfires and earthquakes. Unpleasant.
@mikebarger: Here's the formula:
Propylene glycol ............ 24 ml
DimezoneS/Phenidone ......... 0.2 / 0.105 g (DimezoneS dissolves in 3-5 minutes)
Sodium metaborate 4 mol ..... 6.7 g (dissolves in 3-5 minutes; turns orange)
Ascorbic acid ............... 8.5 g (dissolves in 7-10 minutes; fizzes and turns clear)
Propylene glycol to ......... 33.3 ml (final volume; should need to add little)
Heat to 90C to dissolve everything and drive the water out of the metaborate.
Dissolve in the order shown, and dissolve completely before adding the next chemical.
To make one litre of developer, mix 33.3 ml of concentrate into water containing 90 grams of sodium sulfite. That's 1+29 dilution.
The specific gravity is 1.18, so 33.3 ml of concentrate weighs 39.3 g, letting you measure it by weight if desired.
Times are same as XTOL. Target pH is 8.33.
Mark Overton
Tap-water results
Rudeofus suggested that I try tap-water. Here's the graph of the comparison of XTOL versus the concentrate, using a test-strip of TMY2. The XTOL was dissolved in distilled water, but the concentrate was dissolved in tap-water, so XTOL had an advantage.
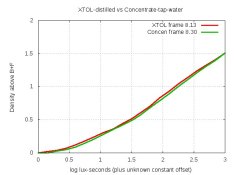
The graphs are very close. Grain is the same, gauging in 22x loupes. My conclusion is: Tap-water had negligible effect.
Also, I saw no evidence of precipitate before or after development, and my water is hard. That's good news, but I'd like to learn more about dealing with dissolved minerals.
@Michael R: I'll confess that I've never tried 1+1 dilution before, so that graph is new to me. With that short toe, I can see why Tri-X is good for pushing.
Mark Overton
Rudeofus suggested that I try tap-water. Here's the graph of the comparison of XTOL versus the concentrate, using a test-strip of TMY2. The XTOL was dissolved in distilled water, but the concentrate was dissolved in tap-water, so XTOL had an advantage.

The graphs are very close. Grain is the same, gauging in 22x loupes. My conclusion is: Tap-water had negligible effect.
Also, I saw no evidence of precipitate before or after development, and my water is hard. That's good news, but I'd like to learn more about dealing with dissolved minerals.
@Michael R: I'll confess that I've never tried 1+1 dilution before, so that graph is new to me. With that short toe, I can see why Tri-X is good for pushing.
Mark Overton
One difficulty is ascorbate is hard to find, so to clone XTOL, one must separately convert some ascorbic acid into ascorbate by adding sodium bicarbonate (baking soda). The problem with that is if the correct amounts of both ingredients are mixed, it will theoretically take a nearly infinite amount of time to convert all the ascorbic acid. To see why, suppose there is one molecule remaining of each ingredient. It will require perhaps years of stirring before those two molecules touch each other. So to complete the conversion in reasonable time, an excess of bicarbonate is needed. But what will that do to the developer?
Mark Overton
Mark, I know you wrote this a while ago, but I've been meaning to comment on it. I would be very surprised if (in general when reacting chemicals together), one needs to place greater quantities of one chemical in order to ensure sufficient reaction completion (assuming reaction rate time constants are not in the order of hours or days). I'm going to assume that the percent of reactant and product both change exponentially w.r.t. time. If so then the reaction will have a time constant (k), such that for a first order reaction,
[A(t)] = [A(0)] exp(kt)
unless k is in the order of hours or days (lets say it is in the order of seconds) then surely you will have sufficient equilibrium after a matter of minutes ? OK I just read here (Summary for reaction orders 0, 1, 2, and n) , that perhaps you have a zero order reaction. Is that the case ? In which case the product concentration only rises linearly not exponentially. Even then I don't imagine you would gain much time advantage by upping a reactant.
Disclaimer: I'm a mathematician and electrical engineer, not a chemist !
Mark, Sodium metaborate 4 mol == NaBO2·4H2O ?
Mark, Sodium metaborate 4 mol == NaBO2·4H2O ?
I'll answer instead of Mark: Photo Formulary sells sodium metaborate which according to their MSDS is sodium metaborate 4 mol. This MSDS lists the formula NaBO2·2H2O [Na2B2O4·4H2O]
Likewise there is a reference on http://www.borax.com which lists both 4 mol (NaBO2·2H2O) and 8 mol (NaBO2·4H2O) sodium metaborate. So for all practical purposes I would assume that Mark means the 4 mol compounds as defined by these references: NaBO2·2H2O
The confusing names seem to come from the double compounds Na2B2O4·4H2O and Na2B2O4·8H2O
thanks, but I have other question - what is the kodalk? )) sodium metaborate 4mol or 8mol?I'll answer instead of Mark
- Joined
- Nov 16, 2004
- Messages
- 3,381
http://www.film-and-darkroom-user.org.uk/forum/archive/index.php/t-5118.html
See the last post.
Ryuji gives Kodalk as 8-mol.This is CAS 1055 76 7
The 4-mol is CAS 16800 11 6
If Kodalk(or other suppliers 8-mol) is used this apparently necessitates a correction for the difference in molecular weight.
Good spot Relayer.
See the last post.
Ryuji gives Kodalk as 8-mol.This is CAS 1055 76 7
The 4-mol is CAS 16800 11 6
If Kodalk(or other suppliers 8-mol) is used this apparently necessitates a correction for the difference in molecular weight.
Good spot Relayer.
So does Grant Haist's book. And if Ryuji and Kodak agree on something, we can assume it's right.Ryuji gives Kodalk as 8-mol.This is CAS 1055 76 7
 *ducks*
*ducks* 
Alan, thanks.
just make some experiment - mix next solution like to Mark formula
Water 1l
Sodium sulfite 90g
Phenidone 0.1g
Ascorbic acid 8.5g
because I haven't metaborate, I replace it with borax+sodium carbonate. 6.7g of Sodium metaborate 4 mol = 6.7/101.83 = 0.066mol. because 1 mol of borax have B4 (metaborate have B1) I take 0.066*381.37/4 = 6.3g of borax Na2B4O7·10H2O. mix and check pH=7.8
after this I add 10% solution of sodium carbonate slowly and check pH. at pH=8.3 I'm stoping and calculate amount of Na2CO3 - its 2.33g/l. so result formula is:
Sodium sulfite 90g
Phenidone 0.1g
Ascorbic acid 8.5g
Borax 6.3g
Sodium carbonate 2.3g
Water 1l
pH=8.3
LegacyPro (Neopan) 400 @400 8min 21C (time from digitaltruth for XTOL stock) - negatives slightly dense than I expected. can't make comparison with XTOL because haven't it. Mark, maybe you can make this formula and test it.
just make some experiment - mix next solution like to Mark formula
Water 1l
Sodium sulfite 90g
Phenidone 0.1g
Ascorbic acid 8.5g
because I haven't metaborate, I replace it with borax+sodium carbonate. 6.7g of Sodium metaborate 4 mol = 6.7/101.83 = 0.066mol. because 1 mol of borax have B4 (metaborate have B1) I take 0.066*381.37/4 = 6.3g of borax Na2B4O7·10H2O. mix and check pH=7.8
after this I add 10% solution of sodium carbonate slowly and check pH. at pH=8.3 I'm stoping and calculate amount of Na2CO3 - its 2.33g/l. so result formula is:
Sodium sulfite 90g
Phenidone 0.1g
Ascorbic acid 8.5g
Borax 6.3g
Sodium carbonate 2.3g
Water 1l
pH=8.3
LegacyPro (Neopan) 400 @400 8min 21C (time from digitaltruth for XTOL stock) - negatives slightly dense than I expected. can't make comparison with XTOL because haven't it. Mark, maybe you can make this formula and test it.
Last edited by a moderator:
@Rudeofus: You were right! An hour or two after I developed film in tap-water, keeping the used developer in a bottle, I noticed some cloudiness formed which became a finely powdered precipitate. I'm doing more experiments to see how long before the precipitate starts forming. If it's at least 30 minutes, it's probably not a problem. Otherwise, I have a problem.  I'm also doing an experiment with metaborate in tap-water to see what happens.
I'm also doing an experiment with metaborate in tap-water to see what happens.
And thanks for answering Relayer's question about 4-mol versus 8-mol. Those names are confusing.
@Relayer: If your developer using borax+carbonate had a pH of 8.3, then I think it should act the same as my developer. The digitaltruth times are starting points, and if it's "slightly dense", then the time was close. I suggest trying 7.5 minutes at 21C. Note that carbonate will not dissolve in propylene glycol.
@PeterB: I think you're correct in that the reaction is 1st-order. But *both* reactants are declining in concentration, which I think results in a 2nd-order reaction and thus a different half-life. I'm a computer scientist, not a chemist, so I'll need to take some time to go through those equations.
Mark Overton
 I'm also doing an experiment with metaborate in tap-water to see what happens.
I'm also doing an experiment with metaborate in tap-water to see what happens.And thanks for answering Relayer's question about 4-mol versus 8-mol. Those names are confusing.
@Relayer: If your developer using borax+carbonate had a pH of 8.3, then I think it should act the same as my developer. The digitaltruth times are starting points, and if it's "slightly dense", then the time was close. I suggest trying 7.5 minutes at 21C. Note that carbonate will not dissolve in propylene glycol.
@PeterB: I think you're correct in that the reaction is 1st-order. But *both* reactants are declining in concentration, which I think results in a 2nd-order reaction and thus a different half-life. I'm a computer scientist, not a chemist, so I'll need to take some time to go through those equations.
Mark Overton
Last edited by a moderator:
richydicky
Thanks for the great work. I have wanted to try XTOL but it would be a waste making 5 litres. I am planning to buy the chemicals tomorrow to make a batch to your formula but I have just checked the supplier list and what they have is 8-mol sodium metaborate. Would appreciate the correction needed for the amount, my chemistry knowledge is over 30 years old!
Results of precipitation-tests in hard tap-water
I mixed 50 ml of developer from sulfite and concentrate, and timed how long it took for visible cloudiness to appear when back-lit by a bare bulb. Temperature was 30C (summer and no A/C), but I think temperature does not affect the rate of precipitation-reactions, so the times below probably won't change with temperature.
My conclusion: With hard water, use the developer immediately after mixing. Or use purified water.
BTW, I examined the test-strip developed with tap-water under the 22x loupe, and saw nothing unusual. I waited around 10 minutes before developing for 9 minutes, so I just missed the beginning of cloudiness. Also, in a different experiment, adding 10 g/L of sodium metaborate to tap-water never produced any cloudiness.
I'll be exploring chelation agents. Thanks again to Rudeofus for suggesting that tap-water be tested. I had overlooked that. Do the chemists here have any comment about these tests and results? Any idea how severe the cloudiness must be before it starts affecting negatives?
Mark Overton
I mixed 50 ml of developer from sulfite and concentrate, and timed how long it took for visible cloudiness to appear when back-lit by a bare bulb. Temperature was 30C (summer and no A/C), but I think temperature does not affect the rate of precipitation-reactions, so the times below probably won't change with temperature.
After 15 minutes: Developer was still perfectly clear.
After 20 minutes: Faint cloudiness visible. It looked clear under normal lighting.
After 30 minutes: More cloudiness, but solution still looked clear under normal lighting.
After one hour: Cloudiness is still staying in suspension (not falling to the bottom). Looks slightly cloudy under normal lighting.
After 20 minutes: Faint cloudiness visible. It looked clear under normal lighting.
After 30 minutes: More cloudiness, but solution still looked clear under normal lighting.
After one hour: Cloudiness is still staying in suspension (not falling to the bottom). Looks slightly cloudy under normal lighting.
My conclusion: With hard water, use the developer immediately after mixing. Or use purified water.
BTW, I examined the test-strip developed with tap-water under the 22x loupe, and saw nothing unusual. I waited around 10 minutes before developing for 9 minutes, so I just missed the beginning of cloudiness. Also, in a different experiment, adding 10 g/L of sodium metaborate to tap-water never produced any cloudiness.
I'll be exploring chelation agents. Thanks again to Rudeofus for suggesting that tap-water be tested. I had overlooked that. Do the chemists here have any comment about these tests and results? Any idea how severe the cloudiness must be before it starts affecting negatives?
Mark Overton
I found original datasheet for Neopan400 from Fuji. 61/4 at 20C and 51/4 at 22C. compare to Kodak 81/4 at 20C. I think that 51/2-53/4 at 21C will be good for next attemptThe digitaltruth times are starting points, and if it's "slightly dense", then the time was close. I suggest trying 7.5 minutes at 21C.
borax and carbonate dissolved in glycerinNote that carbonate will not dissolve in propylene glycol.
Temperature was 30C (summer and no A/C), but I think temperature does not affect the rate of precipitation-reactions, so the times below probably won't change with temperature.
Temperature affects the rates of ALL reactions.
The rule of thumb is that for every 10C increase in temperature, the reaction rate doubles. So a reaction at 30C will be twice as fast as one at 20C.
Also, for precipitation, temperature affects solubility, so that affects the concentration that precipitation begins.
Rule of thumb, solids are more soluble in water as the temperature increases. (The opposite is true for gases dissolved in water.)
Temperature affects the rates of ALL reactions.
The rule of thumb is that for every 10C increase in temperature, the reaction rate doubles. So a reaction at 30C will be twice as fast as one at 20C.
Also, for precipitation, temperature affects solubility, so that affects the concentration that precipitation begins.
Rule of thumb, solids are more soluble in water as the temperature increases. (The opposite is true for gases dissolved in water.)
Kirk, you were right! I re-ran the precipitation-experiment at 20C (instead of 30C), and sure enough, slight cloudiness became visible after 40 min instead of 20 min. That means at 24C, it'll be 30 minutes, so this rule applies to the concentrate:
"If using hard tap-water, develop promptly, making sure development is finished within 30 minutes after mixing."
But I'd like to do better than that, so I'll investigate chelating agents.
I also waited one hour and then developed a test-strip to see what harm this cloudiness does. I'll scan it in 1-2 days.
@Relayer: I haven't forgotten your request to test your modified formula. I'd like to in a few days as time permits.
Mark Overton
thanks.@Relayer: I haven't forgotten your request to test your modified formula. I'd like to in a few days as time permits.
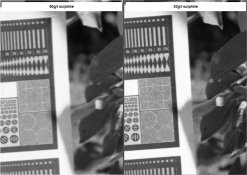
I was still experimenting with my borax-carbonate version. LegacyPro 400 5.5min look ok. my other experiment is decreasing amount of sodium sulphite from 90g/l to 30g/l. amount of borax is same, but I need increase amount of carbonate for correct pH 8.3. formula:
Sodium sulfite 30g
Phenidone 0.1g
Ascorbic acid 8.5g
Borax 6.3g
Sodium carbonate 3.3g
Water 1l
pH=8.3
compared to 90g/l version this developer give less density for same time. more grainly, but sharpness is excellent. sample images for LegacyPro 400 5.5min in attach
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |

