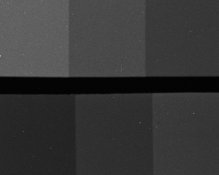
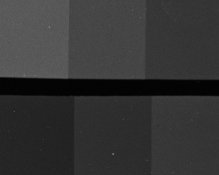
Could folks do me a favor and tell me which crop below is grainier? Even if they look the same to you, please post a note so I'll know:
XTOL:

TEA-mod:

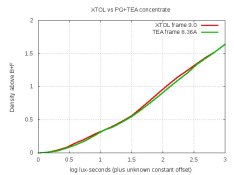
The second is the concentrate with some TEA added, and I'm trying to decide if it's worth pursuing this modification. And please don't laugh about the water-spots and dust; I was casual about cleanliness because these are just test-strips. Here are the density-curves:
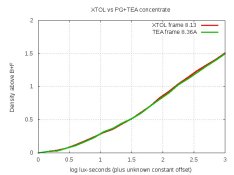
From a density point of view, the modified concentrate is identical to XTOL. But I'm trying to decide about the grain. For the curious, here's the formula. In my test, I dissolved these directly into water:
Propylene glycol .................... 22 ml
TEA85-DEA15 ....................... 3.4 g (yes, that's grams, = 3.1 ml w/ sg=1.107)
Sodium metaborate 4 mol ..... 3.5 g (dissolves in 3-5 minutes. let sit to dry) (4.9 to cnv AA)
DimezoneS/Phenidone .......... 0.2/0.105 g (DimezoneS dissolves in 3-5 minutes. turns orange)
Ascorbic acid ......................... 8 g (dissolves in 7-10 minutes; fizzes and turns clear)
Propylene glycol to ................ 33.3 ml (final volume; should need to add little)
Heat to 90C to dissolve everything and drive the water out of the metaborate.
Dissolve in the order shown, and dissolve completely before adding the next chemical.
To make one litre of developer, mix 33.3 ml of concentrate into water containing 85 grams of sodium sulfite. That's 1+29 dilution.
Times are same as XTOL. Target pH is 8.32.
Concentration ratio = 11.7/33.3 = 0.35
I took the prior concentrate, reduced the ascorbic acid and sulfite a little, and substituted TEA for about half the metaborate. My first test showed that, all other things being equal, TEA gives greater density than metaborate. Alkalis are not created equal. PC likes TEA.
The TEA, labelled "TEA85-DEA15" above, is from Photo Formulary, and their MSDS states that it's 85% TEA and 15% DEA (or slightly more TEA). I tried it a few months ago, and got frustrated because nothing about this TEA agreed with the spec's: specific gravity, pH in solution, and even the freezing point. Whatever is in there, I'm sure it's mostly TEA, but I think of it as "mystery TEA". I'd want to get another sample before recommending this formula. Actually, I'd prefer to use 99% TEA (and boost the 3.4g a little), but that's harder to get in the USA (but easier in Europe).
@athiril: You have an interesting developer using CD-2. Please post that to a new thread where it'll get more visibility.
@Alan Johnson: Thanks for the confirmation. I'm becoming leery of a concentration ratio over 0.35. Maybe I should mix a 0.40 to see if it crystallizes. I'm wondering what the threshold is.
Mark Overton











 But I don't see any degradation in the dark or light areas in the right scan. Nor do I see any issue in a 22X loupe on the light-table. The cloudiness appears to have had no effect. If waiting an hour doesn't hurt anything with hard tap-water, I'm questioning whether it's worth putting a chelating agent into this one-shot formula. I guess I should run more tests to see how severe the cloudiness must be before it affects image-quality.
But I don't see any degradation in the dark or light areas in the right scan. Nor do I see any issue in a 22X loupe on the light-table. The cloudiness appears to have had no effect. If waiting an hour doesn't hurt anything with hard tap-water, I'm questioning whether it's worth putting a chelating agent into this one-shot formula. I guess I should run more tests to see how severe the cloudiness must be before it affects image-quality.