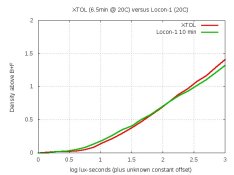
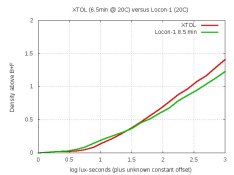
After fighting TMY2 trouble for over a month, I'm finally back trying to formulate developers again.
Today's test was to use no sulfite. But to get finer grain than Gainer's formulas, I kept the pH at 8.0. I tried 100 ml of this 1-litre formula:
Ascorbic acid ...................... 12 g
Sodium metaborate ............ 10.5 g
Dimezone S ........................ 0.20 g
pH = 8.0. Dev'd a strip of TMY2 for 20 minutes at 20C.
The result was a clean-looking negative and purple developer, as you see below:

I suspected that the lack of sulfite let the dyes in the film dissolve, but that purple seems too strong for just 1-1/2 frames. The wash-water only had a very slight magenta tint, so I think most dye was dissolved into the developer.
My densitometer confirms that the neg was thinnish. Also, about one-stop of speed was lost in the shadows.
Questions for the experts:
1. Does sulfite prevent dyes from dissolving into the developer? If so, does that amount of purple look reasonable? The picture matches what I saw.
2. Why does this take so much longer than DS-10 to develop? The pH and Dimezone are the same. Does sulfite remove oxidation-products of the Dimezone? With that not happening, I could see those oxidation-products throttling development.
3. Would using a halide-solvent restore some speed-loss? I've read that sulfite uncovers latent image sites, boosting speed, which obviously isn't happening in this developer.
Mark Overton