albada
Subscriber
Mark; Both the pH and the buffer capacity of those solutions change. You may also include ionic strength in that change. So, by making one ostensible change, you have actually made 3.
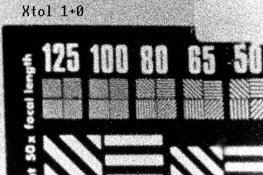
Also, without contrast comparisons, it is hard to judge grain. And, you have to consider sharpness, which may have gone down due to decreasing edge effects. PE
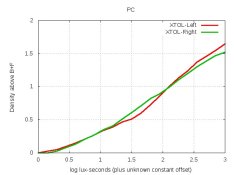
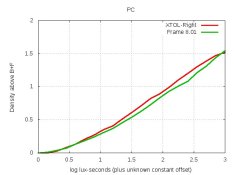
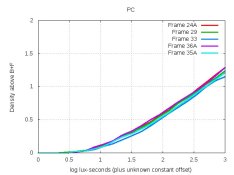
I plotted the curves. Wouldn't they show the effects of those 3 changes on density? Anyway, all curves were quite close (ie, nearly same contrast), so I compared their grain.
But I had not considered sharpness. The test shots of the Stouffer wedge were hand-held, so sharpness-checks must wait for the next roll.
Also, I'm a little surprised that the longer time spent in the sulfite at low pH didn't improve grain. I thought that alone would cause some improvement at low pH. It's odd, it seems that half the things I try surprise me. That tells me how little I understand the development process.
Mark