Good questions, Rudi.
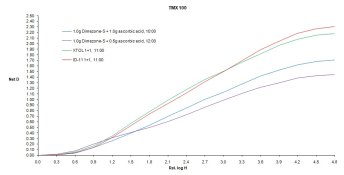
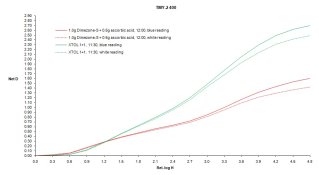
I've been using distilled water, but you are quite right Iron can be introduced by the other ingredients. Thus far I have not had this fail on me, but it is definitely something worth thinking about. On thought I had: when I mixed the "baseline" formula (just 20g/L sulfite and 1g/L Dimezone-S), as expected it went orange during mixing, like POTA does. However all the test formulas which included the "regenerating" superadditive agent (including the latest formula with only 0.5g/L ascorbic acid) mix colourless. So I was thinking, in the case of the ascorbic acid formulas, if Fenton were to show up and destroy the ascorbic acid, I'd expect the solution to go orange. Does this make sense? If so, it would at least be a visible sign of developer death. A way around this entirely would be to go with HQ and adjust the formula. I'm quite certain based on the initial results that can be done. The wild card is image structure. I haven't done any testing on that yet, although I'm hoping to print some tests this weekend.
Regarding Phenidone vs Dimezone-S, my understanding is that if the weights are adjusted, Phenidone and the various available methylated derivatives including Dimezone-S should be expected to have the same photographic characteristics. I don't know if this is correct or not, however. If I had had Phenidone I would have used it. But I happened to have some fresh Dimezone-S so I started these experiments with it and figured I'd continue until it runs out. All this to say there was no special reason for me choosing it over Phenidone.
One thing I'm thinking about is if it would be possibly to replicate the sensitometric results, but with finer grain (assuming it is not fine already, which we don't know) by adjusting the concentrations of the developing agents while increasing the sulfite concentration to say 50g/L for example.
A couple of questions:
1. How do you know when your anhydrous sulfite has crystalized to its hydrated form?
2. While I've experimented briefly with the original Levy POTA formula, I've never tried Delagi's version. What differences do you notice? The only references I have are in Anchell/Troop. My understanding is Delagi modified POTA because he thought it had crap sharpness, but other scientists have said the original POTA is sharper than any of the variants based on it.