You need to be contacting the wedge to the film.
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Progress on XTOL-concentrate
-
A
- Thread starter albada
- Start date
Recent Classifieds
-
For Sale Componon-S 5,6/300 and Componon-S 6,8/360 Enlarging lenses
- Started by alanbradford
-
For Sale Leica, Weston meters and Nikon filters.
- Started by Logical1
-
Want to Buy Fujinon A180mm F/9
- Started by loccdor
-
For Sale FS: Linhof 18x24cm Plate Holders
- Started by B.S.Kumar
-
Under Offer Several Pentax K-mount camera bodies
- Started by madsox
Forum statistics
There's nothing like reading Kodak's instructions! In their publication about B&W films, they say that if magenta remains, the fixer needs to be replaced and that the film should be re-fixed. I did that with my magenta films and the magenta cleared out. The surprise is: Density increased! Both TMY2 and Acros had retained magenta, and here are their graphs before and after re-fixing:
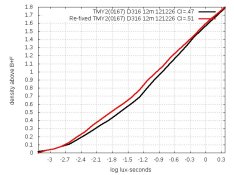
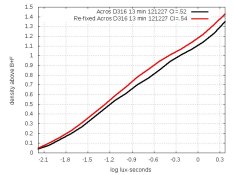
Re-fixed curves are red. I could see why removing dye would decrease fog and thus increase densities by the same amount, but these don't do that. Both of these look like the result of additional development. Densities are still too low, but I don't understand why re-fixing increased them at all.
BTW, when measuring the red TMY2 curve, I used many frames of different exposures of the step-wedge, so there is no run across either row on the step-wedge. Furthermore, I measured Acros the same way, and there is no slope-transition in it. That makes me suspect that the two slopes in TMY2 are really there after all.
Mark Overton


Re-fixed curves are red. I could see why removing dye would decrease fog and thus increase densities by the same amount, but these don't do that. Both of these look like the result of additional development. Densities are still too low, but I don't understand why re-fixing increased them at all.
BTW, when measuring the red TMY2 curve, I used many frames of different exposures of the step-wedge, so there is no run across either row on the step-wedge. Furthermore, I measured Acros the same way, and there is no slope-transition in it. That makes me suspect that the two slopes in TMY2 are really there after all.
Mark Overton
Mark;
You are still removing Dmin, right? The technically valid comparison would include Dmin.
PE
You are still removing Dmin, right? The technically valid comparison would include Dmin.
PE
Mark; You are still removing Dmin, right? The technically valid comparison would include Dmin. PE
Yes, the graphs are density above B+F. But including B+F would merely shift the curves upward; it would not change their shape and slope as we see above. That's what surprised me.
Mark
I have seen curves with Dmin included in which the relative changes cause the curves to become identical when by removing fog they became different. And, since in printing or even just viewing a negative, we see the Dmin, this is a truly correct way of viewing the image in sensitometric curves.
PE
PE
- removed account8
- Deleted
Dmin is base + fog. Dmin is the term used for that by photo engineers just as they use Dmax for the maximum density in a curve.
Lets assume that we have 2 curves which are identical with base + fog removed. Imagine it. Now, add fog and the one with higher fog has higher Dmax as well. This is a 2 way street of deception. It can work the other way too.
The correct way to present a sensitometric curve is with base + fog or with Dmin shown. Sorry, but that is the fact here and the fact that removes any possible error in interpretation.
PE
Lets assume that we have 2 curves which are identical with base + fog removed. Imagine it. Now, add fog and the one with higher fog has higher Dmax as well. This is a 2 way street of deception. It can work the other way too.
The correct way to present a sensitometric curve is with base + fog or with Dmin shown. Sorry, but that is the fact here and the fact that removes any possible error in interpretation.
PE
- removed account8
- Deleted
Michael;
The paper sees the base + fog, and so lets exaggerate this situation to 2 films with a Dmin of 0.2 and 1.0 respectively. Since they are vastly different, you would not print the foggy one at all. Subtracting base + fog might show that they have exactly the same curve shape though, so what might you think? I would probably discard the foggy film shot regardless of the match without for because the foggy example would take 3 stops more exposure to print through the film.
So, in this example, with a foggy film in one case, the example without B+F would appear to match the two curves, but in reality the foggy example is not printable.
Besides, if you slide curves around, as I noted earlier, then you can do the sliding for speed, curve shape, and B+F or minus B+F just by sliding the two sheets of paper.
I use a light table for this type of work.
PE
The paper sees the base + fog, and so lets exaggerate this situation to 2 films with a Dmin of 0.2 and 1.0 respectively. Since they are vastly different, you would not print the foggy one at all. Subtracting base + fog might show that they have exactly the same curve shape though, so what might you think? I would probably discard the foggy film shot regardless of the match without for because the foggy example would take 3 stops more exposure to print through the film.
So, in this example, with a foggy film in one case, the example without B+F would appear to match the two curves, but in reality the foggy example is not printable.
Besides, if you slide curves around, as I noted earlier, then you can do the sliding for speed, curve shape, and B+F or minus B+F just by sliding the two sheets of paper.
I use a light table for this type of work.
PE
Besides, if you slide curves around, as I noted earlier, then you can do the sliding for speed, curve shape, and B+F or minus B+F just by sliding the two sheets of paper. PE
In my graphs 8 postings ago, I slid the curves around (by removing Dmin) to show that:
1. Re-fixing boosted the slope of Acros.
2. Re-fixing changed (and improved) the shape of TMY2.
Why would re-fixing boost slope? Perhaps there was retained silver in thinner areas, as well as retained dye?
Mark Overton
- removed account8
- Deleted
Michael;
We are in a thread on the design of developers and fog is a critical issue (along with curve shape, Dmax, Dmin, and etc....) so we must see all aspect of the curve. This is R&D here not a game. We ned to see all of the data.
Mark;
Fixing or re-fixing can change tone, or the color of the Dmin can change.
PE
We are in a thread on the design of developers and fog is a critical issue (along with curve shape, Dmax, Dmin, and etc....) so we must see all aspect of the curve. This is R&D here not a game. We ned to see all of the data.
Mark;
Fixing or re-fixing can change tone, or the color of the Dmin can change.
PE
Fixing or re-fixing can change tone, or the color of the Dmin can change. PE
I got the Dmin numbers:
Before re-fixing the TMY2 (ie, with magenta cast), Dmin = 0.40.
After re-fixing, Dmin = 0.26, which is typical of this film.
That's a drop of 0.14. Visually, I'd say the removal of the dye can explain this entire drop.
@Michael: There's no problem about discussing inclusion of B+F in graphs. I just didn't want my question about slope-change to be forgotten.
Mark
- removed account8
- Deleted
Michael, apologies to you and others. I know that to some people, the quality of their developers or processing is not a serious proposition. I myself take it very seriously and do my measurements to the same standards I used at EK. This actually included B+F or with Dmin and without B+F depending on the purposes of the work.
My work area is messy, but very very well equipped. I even graph on Kodak paper as can be seen in some of my posts. I''m used to making my measurements and calculations from those curves.
PE
My work area is messy, but very very well equipped. I even graph on Kodak paper as can be seen in some of my posts. I''m used to making my measurements and calculations from those curves.
PE
Mark - is the Dmin of 0.26 with your developer or XTOL? Just asking because I usually get slightly lower Dmin with TMax films with XTOL (and other developers). Usually 0.20 +/-.02. Then again it could just be a difference in densitometers.
The TMY2 Dmin of 0.26 is with my developer, D316. XTOL consistently gives me a Dmin of 0.25. Yes, I think this could be a difference in our densitometers. One of them could have a slight nonlinearity around the calibration-setting for pure white.
Mark
D316 is a two-part developer consisting of sodium sulfite powder and a liquid concentrate. I'm finding that it's convenient to measure both by weight. Electronic scales are cheap. Search on amazon for "scale .01g". They typically cost about $15, and work well in my experience. That's the cost of just three rolls of film. At that cost, there's no excuse for a darkroom to not have one. To mix this dev:
D316 weighs 22.8 grams/litre. And you need 45 grams/litre of sulfite. So to make 250 ml of dev, pour out 5.7 g of liquid and 11.3 g of sulfite.
I prefer to do this than deal with air in bottles of XTOL plus the initial mixing of 5L of XTOL. All things considered, D316 is not much less convenient than XTOL. It's fine for occasional shooters of 1-2 rolls/month for whom the few extra minutes of measuring and stirring won't matter.
Mark Overton
1. Put a plastic beaker on the scale and press Tare to zero it.
2. Pour in the correct weight of liquid.
3. Press Tare to re-zero the scale.
4. Pour in the correct weight of sulfite.
5. Add water to fill-line and stir for 3.5 minutes to dissolve.
2. Pour in the correct weight of liquid.
3. Press Tare to re-zero the scale.
4. Pour in the correct weight of sulfite.
5. Add water to fill-line and stir for 3.5 minutes to dissolve.
D316 weighs 22.8 grams/litre. And you need 45 grams/litre of sulfite. So to make 250 ml of dev, pour out 5.7 g of liquid and 11.3 g of sulfite.
I prefer to do this than deal with air in bottles of XTOL plus the initial mixing of 5L of XTOL. All things considered, D316 is not much less convenient than XTOL. It's fine for occasional shooters of 1-2 rolls/month for whom the few extra minutes of measuring and stirring won't matter.
Mark Overton
Mark;
I'll bet that some would find it convenient to have the formula and the mixing instructions in one long post that they can cut and paste for their lab. Could you please do that.
I know it would be nice for my own work.
Thanks.
PE
I'll bet that some would find it convenient to have the formula and the mixing instructions in one long post that they can cut and paste for their lab. Could you please do that.
I know it would be nice for my own work.
Thanks.
PE
- removed account8
- Deleted
Mark; I'll bet that some would find it convenient to have the formula and the mixing instructions in one long post that they can cut and paste for their lab. Could you please do that. I know it would be nice for my own work. Thanks. PE
Wow; it's been awhile since I posted the formula. Here it is:
Propylene glycol ................. 16 ml
Sodium metaborate 4 mol ..... 2.2 g
Ascorbic acid ..................... 4.5 g
Phenidone ......................... 0.05 g
I find it useful to multiply this formula by 3 and store in a little 64-ml bottle sold by the Formulary. I suggest *not* using a dropper-cap because they let more oxygen in.
Mix everything at about 75C, stirring continuously. Dissolve each ingredient before adding the next, except that Phenidone may be added when most but not all of the ascorbic acid has been dissolved. It only takes a few minutes to dissolve each ingredient. It can be hard to tell if dissolving is complete because there are many tiny bubbles that look like particles of powder. Stop stirring and if they tend to float upward, you're done.
To use, weigh into a beaker at the rates of 22.8 g/L of concentrate and 45 g/L of sodium sulfite, and then add water. Stirring for 3.5 min at 20C will dissolve all the sulfite.
Here are times in minutes at 20C:
Tmax-100 13.75
Tmax-400 12
Tri-X 13
Acros 13.5
Neopan-400 16.5
PanF+ 12.25
FP4+ 16
HP5+ 14
Delta-100 13
Delta-400 17
Delta-3200 25
These times might change a little as I do some re-testing.
"D316" is a temporary name. I'm thinking of calling it "KF-1" where "KF" means "keep frozen". It stores fine in the freezer, and I'll speculate that it'll last at least two years frozen.
I'm investigating reducing both the metaborate and ascorbic acid. This might allow us to use Dimezone S without crystallization.
Mark
EDIT: To put everything in one posting, I should have added the following about the results.
Compared with XTOL, this developer:
* Has the same grain or a hint finer.
* Sharpens a little less.
* Has the same density-curve, but with a slightly higher shoulder, and the toe might be slightly softer, but the difference is so small that it's hard to tell.
Last edited by a moderator:
Thanks. I'm sure everyone will enjoy this.
PE
PE
Thanks. I'm sure everyone will enjoy this. PE
Ron or anyone else:
If you decide to mix this, could you weigh the beaker, thermometer and stirring rod, both before starting and after finishing the mixing? The difference between those pre- and post-weights will be the total weight of concentrate you got. It will include the carry-out loss on the thermometer and rod, so it should be accurate. Divide that by whatever you multiplied the formula by (I suggest 3x), and you get the g/L of concentrate to use. It should be about 23 g/L. Whatever number you get, that's the number you should use when using the developer. I'm curious what number somebody else gets after careful measuring.
I just checked the accuracy of the syringe I use for measuring propylene glycol (PG), and discovered that it's just over 1% short. A more accurate weight of concentrate should be more like 23.1 g/L instead of 22.8. But for normal usage, specifying "use 23 g/L of concentrate" will be plenty accurate.
Note that the weight of raw ingredients is 23.326 g, using a sg of 1.036 for PG. With the syringe-correction, mine weighs 23.1 g. That's a loss of about 0.2 g, which should be the weight of PG and water that got steamed out. Some PG is lost through vapour, so water-loss is even less than 0.2 g. But 2.2 g of sodium metaborate 4 mol contains 0.8 g of water. That tells me little water is steamed-out. So I suspect D316 could be mixed at room-temperature, although it would take hours or perhaps days to dissolve it all. And you can forget about steaming-out water (referring to the discussion about this a few weeks ago).
Mark
Mark, thanks for the repost of the entire formula. I'm gearing up to process some TMY this weekend.
FWIW, I like the name D316. Makes me feel like being in on a secret.
-- Jason
FWIW, I like the name D316. Makes me feel like being in on a secret.
-- Jason
What's a Mol? As in: Sodium metaborate 4 mol 2.2 g
Is this a different Sodium metaborate than found at Photographers Formulary?
You may think this a dumb question and perhaps it is, but ... I want to be sure I have the correct chemistry.
Thanks for your efforts, they are appreciated.
Is this a different Sodium metaborate than found at Photographers Formulary?
You may think this a dumb question and perhaps it is, but ... I want to be sure I have the correct chemistry.
Thanks for your efforts, they are appreciated.
@kb3lms: If I recall, you used a different amount of PG when mixing, so you'll use a different weight from the 23 g/L I mentioned above. Of course, you can measure by volume instead. The different PG also might mean that your dev-time will be a little different from mine. Let us know how it goes.
Bruce, actually, you bring up an interesting question. Folks get confused because the names of sodium metaborate changed decades ago as folks got a better understanding of its structure. The old and new names don't match, causing confusion. Sodium metaborate 4 mol is also called "sodium metaborate dihydrate" because each molecule has two water molecules attached to it. Yes, the Formulary sells the correct kind, and in fact I bought mine from the Formulary.
Mark Overton
What's a Mol? As in: Sodium metaborate 4 mol 2.2 g
Is this a different Sodium metaborate than found at Photographers Formulary?
You may think this a dumb question and perhaps it is, but ... I want to be sure I have the correct chemistry.
Thanks for your efforts, they are appreciated.
Bruce, actually, you bring up an interesting question. Folks get confused because the names of sodium metaborate changed decades ago as folks got a better understanding of its structure. The old and new names don't match, causing confusion. Sodium metaborate 4 mol is also called "sodium metaborate dihydrate" because each molecule has two water molecules attached to it. Yes, the Formulary sells the correct kind, and in fact I bought mine from the Formulary.
Mark Overton
Do you have any trouble measuring the
Phenidone ......................... 0.05 g
even times three that's pretty small. Where I buy my Phenidone they indcate they will not measure out less than .5
I don't mix much from scratch....just a question.
Thanks
Phenidone ......................... 0.05 g
even times three that's pretty small. Where I buy my Phenidone they indcate they will not measure out less than .5
I don't mix much from scratch....just a question.
Thanks
Make the phenidone up at 10x in alcohol. That would be 1/2 gram say in 100 ml of alcohol. Then add 1 ml of this to the concentrate.
And guys, always weigh out viscous liquids. Never measure by volume.
PE
And guys, always weigh out viscous liquids. Never measure by volume.
PE
On the PG, I ended up with a slightly different amount. I don't remember why. Since I had multiplied the formula to use 0.25g phenidone (smallest I was comfortable weighing) I think it had something to do with that. Anyway I had used a slightly different amount of concentrate to make 1 liter and the pH was slightly off. We had decided that I should have used the correct amount. I also used the 8 mol variety Sodium Metaborate.
I can report that the small bottle of concentrate is sitting on my shelf entirely unchanged from the day I mixed it. I can also report that I tried developing a few frames of homebrew emulsion (TLF2) in it and the contrast was quite low compared to D-23 or D-76 1:1. However, the contrast of that batch of emulsion was low anyway compared to other batches I had made, which are all lower contrast than commercial films.
I like the idea about dissolving the phenidone in alcohol and measuring it out that way.
Jason
I can report that the small bottle of concentrate is sitting on my shelf entirely unchanged from the day I mixed it. I can also report that I tried developing a few frames of homebrew emulsion (TLF2) in it and the contrast was quite low compared to D-23 or D-76 1:1. However, the contrast of that batch of emulsion was low anyway compared to other batches I had made, which are all lower contrast than commercial films.
I like the idea about dissolving the phenidone in alcohol and measuring it out that way.
Jason
Make the phenidone up at 10x in alcohol. That would be 1/2 gram say in 100 ml of alcohol. Then add 1 ml of this to the concentrate.
And guys, always weigh out viscous liquids. Never measure by volume.
PE
Anyone have accurate numbers for the weight of 6 ml of HC110 concentrate
 ?
?Anyone have accurate numbers for the weight of 6 ml of HC110 concentrate?
I know you're mostly joking, but I went ahead weighed a 6 ml syringe empty and full of HC110, and subtracted, which gave: 7.2 g.
But the ml and weight numbers printed on the side of the bottle work out to 6.3 g.
So something is wrong.
Mark
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |

