As I mentioned last week, I'm trying variations of a baseline developer. Here are six developers I recently tested:
Sodium sulfite ............... 35, 40, 45, 47.5, 50, 55 g
Sodium metaborate ...... 2.4, 2.2, 2.0, 1.9, 1.8, 1.6 g
Ascorbic acid ................ 4.5 g
Phenidone .................... 0.05 g
Propylene glycol ........... 15.5 ml (includes %-solution for Phenidone)
pH = 8.02 for all of these.
All were developed to the same contrast (slope) as stock XTOL for TMY2.
The experiment was to see what effect various levels of sulfite had, keeping pH constant. I'll identify each developer by its sulfite content (e.g., "the 40 developer").
The grain looks identical for the 40 through 55 developers, and is a hint better than XTOL. Grain is a hint coarser than XTOL for the 35 developer. The lower sulfite developers might be a tad sharper than the highest sulfite developers.
The dev-times were: 15.3, 14.25, 14.1, 14.0, 13.83, 13.58 minutes. Here's a graph of those dev-times:
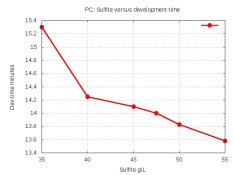
The pH is constant, so the time-differences are solely due to the synergistic effect of sulfite on the developer. Notice that dev-time shoots up when sulfite drops below 40.
Here's the most interesting graph. This shows loss of true speed (Y-axis) versus sulfite-content (X-axis):
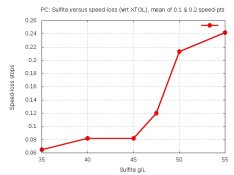
For each developer, I calculated the difference in EI (i.e., effective film-speed) using speed-points determined by densities of .1 above B+F and .2 above B+F, and calculated the loss of speed compared with XTOL. I averaged the number-pairs to help smooth the results, resulting in this chart.
Yes, XTOL is always faster than these developers, I suspect because it uses DimezoneS instead of Phenidone. But the loss is insignificant up to 45 -- only 0.08 stops of speed lost; that's only 1/12th of a stop. But above 45, the loss shoots up. So adding sulfite over 45 reduces true speed and gains nothing that I can see.
Evaluating trade-offs: There's no advantage in going above 45; all that does is reduce speed and possibly sharpness. And 35 gives worse grain with an insignificant speed-boost (and longer dev-time). That leaves 40 and 45. Suppose the user of this developer is not accurate in measuring sulfite. Then selecting 40 means risking thin neg's if too little sulfite is supplied. OTOH, 45 is more tolerant of too little, but risks a small speed-loss if too much sulfite is supplied. However, in this case the higher pH will partly compensate by increasing density. So the risk of disappointment is lower with 45 than 40. So I think 45 wins. That happens to be the baseline developer I described here: (there was a url link here which no longer exists).
Any comments about all this?
I'm thinking of boosting metaborate to boost pH and see what happens. The low pH of 8.0 concerns me because DS-10 had trouble with some slow films, and its pH was 8.0, which might be the cause. The amount of ascorbic acid seems a little high, as the ascorbic/phenidone ratio is 90. Gainer's graph leveled off at 80. That suggests either decreasing my ascorbic acid or increasing the phenidone. Any suggestions about other variations to try?
Mark Overton
Sodium sulfite ............... 35, 40, 45, 47.5, 50, 55 g
Sodium metaborate ...... 2.4, 2.2, 2.0, 1.9, 1.8, 1.6 g
Ascorbic acid ................ 4.5 g
Phenidone .................... 0.05 g
Propylene glycol ........... 15.5 ml (includes %-solution for Phenidone)
pH = 8.02 for all of these.
All were developed to the same contrast (slope) as stock XTOL for TMY2.
The experiment was to see what effect various levels of sulfite had, keeping pH constant. I'll identify each developer by its sulfite content (e.g., "the 40 developer").
The grain looks identical for the 40 through 55 developers, and is a hint better than XTOL. Grain is a hint coarser than XTOL for the 35 developer. The lower sulfite developers might be a tad sharper than the highest sulfite developers.
The dev-times were: 15.3, 14.25, 14.1, 14.0, 13.83, 13.58 minutes. Here's a graph of those dev-times:

The pH is constant, so the time-differences are solely due to the synergistic effect of sulfite on the developer. Notice that dev-time shoots up when sulfite drops below 40.
Here's the most interesting graph. This shows loss of true speed (Y-axis) versus sulfite-content (X-axis):

For each developer, I calculated the difference in EI (i.e., effective film-speed) using speed-points determined by densities of .1 above B+F and .2 above B+F, and calculated the loss of speed compared with XTOL. I averaged the number-pairs to help smooth the results, resulting in this chart.
Yes, XTOL is always faster than these developers, I suspect because it uses DimezoneS instead of Phenidone. But the loss is insignificant up to 45 -- only 0.08 stops of speed lost; that's only 1/12th of a stop. But above 45, the loss shoots up. So adding sulfite over 45 reduces true speed and gains nothing that I can see.
Evaluating trade-offs: There's no advantage in going above 45; all that does is reduce speed and possibly sharpness. And 35 gives worse grain with an insignificant speed-boost (and longer dev-time). That leaves 40 and 45. Suppose the user of this developer is not accurate in measuring sulfite. Then selecting 40 means risking thin neg's if too little sulfite is supplied. OTOH, 45 is more tolerant of too little, but risks a small speed-loss if too much sulfite is supplied. However, in this case the higher pH will partly compensate by increasing density. So the risk of disappointment is lower with 45 than 40. So I think 45 wins. That happens to be the baseline developer I described here: (there was a url link here which no longer exists).
Any comments about all this?
I'm thinking of boosting metaborate to boost pH and see what happens. The low pH of 8.0 concerns me because DS-10 had trouble with some slow films, and its pH was 8.0, which might be the cause. The amount of ascorbic acid seems a little high, as the ascorbic/phenidone ratio is 90. Gainer's graph leveled off at 80. That suggests either decreasing my ascorbic acid or increasing the phenidone. Any suggestions about other variations to try?
Mark Overton





