Great stuff, Mark! Keep going!
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Progress on XTOL-concentrate
-
A
- Thread starter albada
- Start date
Recent Classifieds
-
For Sale Zeiss 50mm f1.5 Sonnar ZM (Leica M) (Boxed Silver)
- Started by jshcrlsn
-
Want to Buy Hasselblad bits & pieces
- Started by eli griggs
-
For Sale 1961 Leica Summilux 50mm ver 2
- Started by Oldwino
-
For Sale Near Mint 1956 Leica M3 DS / 1956 Summicron 5cm
- Started by Oldwino
-
Want to Buy B+W 49mm yellow-green 060 filter
- Started by Kodachromeguy
Forum statistics
Mark;
If you repeated that experiment several times, I would guess that these differences would even out and the averages would be virtually identical.
One maxim I learned was "never do any experiment one time".
PE
If you repeated that experiment several times, I would guess that these differences would even out and the averages would be virtually identical.
One maxim I learned was "never do any experiment one time".
PE
Mark;
If you repeated that experiment several times, I would guess that these differences would even out and the averages would be virtually identical.
One maxim I learned was "never do any experiment one time".
PE
The speed-loss of that last graph is only 1/6th of a stop. As both you and Rudi suggest, this is tiny and may be solely due to normal experimental variation. I only did one experiment at that ratio of 117. The problem is, it takes me a couple of hours to run one experiment. My steps are:
- put a film-strip in tank, held down with magnets.
- measure and mix chemicals to make 100 ml of test-brew (slow when adjusting pH to a target at 20C).
- bring developer and water-bath for the tank to 20C.
- develop, stop, fix, wash, dry.
- measure densities w/ densitometer.
- type them into computer and graph them.
- if it looks promising: compare grain/sharpness with loupes, and scan strip into computer.
- ponder what to try next.
As a result, one experiment is one evening. So an array of tests, such as trying various amounts of some chemical, means using an array of evenings, and/or all day Saturday. And that's doing each experiment just once. And that's doing just strips, not whole rolls. Back in the day, did Kodak's engineers have testers or technicians available to do much of this tedious work for them? If so, that must have been great.
I'm thinking it might be worthwhile to run longer strips containing two frames instead of just one. That may give most of the advantages of two identical experiments, yet consuming little extra time. Any thoughts about how to speed up experiments?
@kb3lms: We're getting closer! The formula is now fairly stable, so now I'm thinking of things like chelation and improving convenience.
Mark Overton
Mark;
I fully understand. I was just making a point regarding that tiny difference. I would consider it a non-difference!
PE
I fully understand. I was just making a point regarding that tiny difference. I would consider it a non-difference!
PE
Another question for those who know chemistry:
How much citric acid is needed to chelate calcium and magnesium in hard water?
My analysis below says 1 g/L. But is this analysis correct?
Water is consided "very hard" if it contains over 180 ppm of metal ions. Let's assume we have 200 ppm. That's .2 g/L. Let's assume it's all calcium. The atomic weight of calcium is 40 g/mole. So have .2/40 moles/L of calcium in the water. Citric acid has a molecular weight of 192 g/mole. And assume each molecule of citric acid can chelate one atom of calcium. That means we need (.2/40)*192 = 0.96 g/L of citric acid to chelate that calcium.
Magnesium is lighter, weighing 24 g/mole, so we'd need (.2/24)*192 = 1.6 g/L of citric acid to chelate solely magnesium. But I'll assume that hard water contains mostly calcium (is this true?), so closer to 1 g/L of citric acid is sufficient.
Or did I goof?
BTW, I'm ignoring the problem of the Fenton reaction with iron which slowly destroys the ascorbate. That reaction occurs over days or weeks. My immediate goal is to prevent the developer from becoming cloudy after 45 minutes due to calcium and magnesium precipitating out of hard water. Also, to maintain pH, the citric acid will need to be countered by considerably more sodium metaborate, which will improve buffering.
Mark Overton
How much citric acid is needed to chelate calcium and magnesium in hard water?
My analysis below says 1 g/L. But is this analysis correct?
Water is consided "very hard" if it contains over 180 ppm of metal ions. Let's assume we have 200 ppm. That's .2 g/L. Let's assume it's all calcium. The atomic weight of calcium is 40 g/mole. So have .2/40 moles/L of calcium in the water. Citric acid has a molecular weight of 192 g/mole. And assume each molecule of citric acid can chelate one atom of calcium. That means we need (.2/40)*192 = 0.96 g/L of citric acid to chelate that calcium.
Magnesium is lighter, weighing 24 g/mole, so we'd need (.2/24)*192 = 1.6 g/L of citric acid to chelate solely magnesium. But I'll assume that hard water contains mostly calcium (is this true?), so closer to 1 g/L of citric acid is sufficient.
Or did I goof?
BTW, I'm ignoring the problem of the Fenton reaction with iron which slowly destroys the ascorbate. That reaction occurs over days or weeks. My immediate goal is to prevent the developer from becoming cloudy after 45 minutes due to calcium and magnesium precipitating out of hard water. Also, to maintain pH, the citric acid will need to be countered by considerably more sodium metaborate, which will improve buffering.
Mark Overton
Mark;
Citric Acid is a poor chelaing agent. This means that 1 mole of CA does not complex with 1 mole of Ca++.
Now, assuming that 1 mole of Ca++ = 1 mole of CA, then your figures are ok, but you need an excess. If you look at the sequestrant levels in most developers, you find that it is about 2 - 5 g/l for strong sequestrants.
PE
Citric Acid is a poor chelaing agent. This means that 1 mole of CA does not complex with 1 mole of Ca++.
Now, assuming that 1 mole of Ca++ = 1 mole of CA, then your figures are ok, but you need an excess. If you look at the sequestrant levels in most developers, you find that it is about 2 - 5 g/l for strong sequestrants.
PE
Mark;
If you repeated that experiment several times, I would guess that these differences would even out and the averages would be virtually identical.
One maxim I learned was "never do any experiment one time".
PE
You guys were right! I re-ran the experiment this evening, but boosting the AA even more, which should have cut the speed even more if my theory were correct. Instead, I got this curve:

This graph thinks the film-speed is a hair *higher*. I stand corrected: the amount of AA has no effect on speed.
Mark Overton
This graph thinks the film-speed is a hair *higher*. I stand corrected: the amount of AA has no effect on speed.
That's the reason why I wanted to know at which level speed or other properties deviate by more substantial amounts. I assume you have the data right in front of you from the many experiments you have done by now. If you look at Pat Gainer's results (here and here), you shouldn't expect much difference even with big changes in AA amount.
Today I did what Microsoft calls, "Eating your own dog-food." I developed an important roll in my own developer. Here's the latest concentrate:
Compared to earlier formulas, I boosted metaborate and ascorbic acid to improve buffering in order to make the developer more tolerant of errors in measurement of sodium sulfite. To make 1 liter of working solution, first mix 30 ml (45 grams) of sodium sulfite into the water. Then add 30 ml of concentrate. As a convenience, the dev requires the same volume of sulfite and concentrate (30 ml/L each), so you need to draw only one fill-line on your little beaker. pH is 8.15 to 8.17. Time at 20C is about 1.9 times XTOL's time.
I was frugal and mixed only 200 ml of solution, the bare minimum for a SS tank, and developed one roll of TMY-2 (Tmax-400). The results are best described as "strong": Leader-density is 3.0 to 3.15, and edge-markings are a little denser than XTOL's. In my past experience, developing a full roll (36-shots) resulted in lower density than in test-strips. This dev broke that rule: The roll is just as dense, or a little denser. Perhaps this is due to the strong buffering.
By the way, I pre-washed the roll, and I always pre-wash test-strips. I discovered in March that TMY-2 gives me higher density when I pre-wash it. You can find discussion of this in older threads.
The event was a wedding, and I noticed two men in the parking lot fixing a van:

And here's a full-resolution crop of the neg-scan:

The grain is plenty fine. As I saw in test-strips, this dev looks like XTOL. So I'm getting close. The formula above needs a tweak or two, and much more testing. Comments are welcome as always. A question that I'm not entirely confident about is whether it's worth boosting the buffering as I did. Or is that just a waste of chemistry?
Mark Overton
Propylene glycol ......................... 23 ml
Sodium metaborate (4 mol) ....... 4.7 g
Ascorbic acid ............................. 6.5 g
Phenidone ................................. 0.05 g
Propylene glycol to make .......... 30 ml
Sodium metaborate (4 mol) ....... 4.7 g
Ascorbic acid ............................. 6.5 g
Phenidone ................................. 0.05 g
Propylene glycol to make .......... 30 ml
Compared to earlier formulas, I boosted metaborate and ascorbic acid to improve buffering in order to make the developer more tolerant of errors in measurement of sodium sulfite. To make 1 liter of working solution, first mix 30 ml (45 grams) of sodium sulfite into the water. Then add 30 ml of concentrate. As a convenience, the dev requires the same volume of sulfite and concentrate (30 ml/L each), so you need to draw only one fill-line on your little beaker. pH is 8.15 to 8.17. Time at 20C is about 1.9 times XTOL's time.
I was frugal and mixed only 200 ml of solution, the bare minimum for a SS tank, and developed one roll of TMY-2 (Tmax-400). The results are best described as "strong": Leader-density is 3.0 to 3.15, and edge-markings are a little denser than XTOL's. In my past experience, developing a full roll (36-shots) resulted in lower density than in test-strips. This dev broke that rule: The roll is just as dense, or a little denser. Perhaps this is due to the strong buffering.
By the way, I pre-washed the roll, and I always pre-wash test-strips. I discovered in March that TMY-2 gives me higher density when I pre-wash it. You can find discussion of this in older threads.
The event was a wedding, and I noticed two men in the parking lot fixing a van:

And here's a full-resolution crop of the neg-scan:

The grain is plenty fine. As I saw in test-strips, this dev looks like XTOL. So I'm getting close. The formula above needs a tweak or two, and much more testing. Comments are welcome as always. A question that I'm not entirely confident about is whether it's worth boosting the buffering as I did. Or is that just a waste of chemistry?
Mark Overton
Mark, thank you for working on this developer. I have been following your discoveries with great interest, as XTol 1+1 is the only film developer I use. I was wondering if you had any data, by now, about the keeping properties of the concentrate, since quite some time has passed since you begun.
Many thanks, and good luck.
Many thanks, and good luck.
- Joined
- Nov 16, 2004
- Messages
- 3,400
See post no. 10 here:
(there was a url link here which no longer exists)
Stock solutions mixed in glycol should last at leat 2-3 years.
But Xtol concentrate contains some water of crystallisation from the metaborate.
I am running a 1 year test on early Xtol concentrate D-316 due to complete Jan 2013 which should show if this water of crystallisation has an adverse effect on shelf life.Even if it does it should be possible to make a recommendation to heat the concentrate when it is made, to drive off this water.
(there was a url link here which no longer exists)
Stock solutions mixed in glycol should last at leat 2-3 years.
But Xtol concentrate contains some water of crystallisation from the metaborate.
I am running a 1 year test on early Xtol concentrate D-316 due to complete Jan 2013 which should show if this water of crystallisation has an adverse effect on shelf life.Even if it does it should be possible to make a recommendation to heat the concentrate when it is made, to drive off this water.
Good work Mark;
PE
PE
Mark, thank you for working on this developer. I have been following your discoveries with great interest, as XTol 1+1 is the only film developer I use. I was wondering if you had any data, by now, about the keeping properties of the concentrate, since quite some time has passed since you begun.
Many thanks, and good luck.
Firstly: Alan: Thanks for doing the D-316 testing, and for the link about storage. I hadn't seen those postings.
Rafal, I have no results from long term tests yet. But I have some short term failures. For example, in one experiment, a concentration-ratio of 0.46 resulted in crystallization after a couple of weeks. I'm now testing a ratio of 0.40. The purpose of these ratio-experiments is to see how high concentration can be.
The concentrate (and sodium sulfite) will last longer if stored in the refrigerator or freezer. A few months ago, I put some concentrate in the freezer, and got no precipitation. If frozen, I would expect it to last for many years. Of course, you must remember to take the two bottles out of the freezer a few hours before you need them.
Mark Overton
Here's a question that perhaps only PE can answer (but anyone is welcome to try):
The curves below show two XTOL strips averaged together (red), and two strips from the formula used for the wedding (green and blue):

Note the right quarter of the graphs. The red XTOL graph rises only slighly in the right quarter, but the two wedding graphs have a more pronounced rise there. My earlier developers with less buffering (and AA) don't do this. But I do see it in one earlier dev with more buffering (and AA), as well as the latest two strips. So I think that right-rise is real. I'm guessing it's caused by either (1) boosted buffering, or (2) boosted ascorbic acid (AA) which is a secondary developer. This developer is rather dilute, so I'm surprised to see a boost in highlights instead of compensation that one normally expects with dilution.
Any idea why boosting buffering or AA would cause highlights to have higher slope than shadows and midtones?
Can the curve be linearized?
I was thinking of adding KBr, but that's not soluble in PG. Maybe benzoic acid? Other ideas?
I know the right-rise is small, but I'm a perfectionist who'll go for linear if possible.
Thanks,
Mark Overton
The curves below show two XTOL strips averaged together (red), and two strips from the formula used for the wedding (green and blue):

Note the right quarter of the graphs. The red XTOL graph rises only slighly in the right quarter, but the two wedding graphs have a more pronounced rise there. My earlier developers with less buffering (and AA) don't do this. But I do see it in one earlier dev with more buffering (and AA), as well as the latest two strips. So I think that right-rise is real. I'm guessing it's caused by either (1) boosted buffering, or (2) boosted ascorbic acid (AA) which is a secondary developer. This developer is rather dilute, so I'm surprised to see a boost in highlights instead of compensation that one normally expects with dilution.
Any idea why boosting buffering or AA would cause highlights to have higher slope than shadows and midtones?
Can the curve be linearized?
I was thinking of adding KBr, but that's not soluble in PG. Maybe benzoic acid? Other ideas?
I know the right-rise is small, but I'm a perfectionist who'll go for linear if possible.
Thanks,
Mark Overton
Mark;
If you are at the limit of the buffer's capacity (which can take place at the densest part of the negative) then you can get a downdurn. This may be a case of film and developer being built together to give the proper curve and image quality and therefore your developer here is overdoing some aspect that should be more restrained.
If that is the case, then I would expect some degradation in the image structure in your developer wrt the XTOL.
That is what I am guessing at this stage. Your developer is overdoing things and should have less buffer. In its present state, your developer will suffer in grain or sharpness or both wrt XTOL.
PE
If you are at the limit of the buffer's capacity (which can take place at the densest part of the negative) then you can get a downdurn. This may be a case of film and developer being built together to give the proper curve and image quality and therefore your developer here is overdoing some aspect that should be more restrained.
If that is the case, then I would expect some degradation in the image structure in your developer wrt the XTOL.
That is what I am guessing at this stage. Your developer is overdoing things and should have less buffer. In its present state, your developer will suffer in grain or sharpness or both wrt XTOL.
PE
Your developer is overdoing things and should have less buffer.
Thanks for the response. That dev has both high buffering and a large AA/Phenidone ratio, so I'm thinking of running experiments (evenings...) to separate those two factors. "Overdoing things". The purpose of that was to tolerate errors in volumemetric sulfite-measurement, and that made me think of...
----- Here's an idea for measurement -----
I thought of a way to accurately measure sodium sulfite by volume, using a typical little beaker or medicine cup:
Step 1: Pour the correct amount of liquid concentrate into the cup (up to a fill-line).
Step 2: Add sulfite until the liquid reaches a second higher fill-line.
Step 3: Pour the cup into the main beaker containing water and mix.
This works because the liquid drives out the air between the sulfite particles, so you're only measuring the volume of solid. I tried this using only propylene glycol (PG) in a graduated beaker. I first poured in some PG, then added a known weight of sulfite, and measured the level. This let me measure the specific gravity of sulfite, and it was accurate within 1%! Success! Except...
The problem with this method is the sodium sulfite becomes a sticky glob in the bottom of the cup, perhaps due to trace water in the PG. It won't pour out, and must be helped with a popsicle-stick or somesuch. To get it all out, the little cup should then be rinsed in the beaker (dipped a few times). Would folks be willing to do this?
----- Here's a 2-bath idea -----
Since these developers consist of concentrate and separate sulfite, I realized they could also do double-duty as two-bath developers. A normal A-then-B development would be too thin, so you'd cycle back and forth between A-bath and B-bath a few times, spending 2-3 minutes in each bath. You should get compensating development typical of two-baths.
Cycling back and forth will contaminate each bath a little, but that won't matter because these developers are one-shot. Also, you'll need to mix say 50% more dev in each bath, and develop using the A-B cycling for a longer total time.
It would be appealing to have one developer that can be used either as a conventional single-bath or as a two-bath.
I've got more ideas than time to experiment with them. Retirement is sounding good!
Comments on all this are encouraged.
Mark Overton
The wedding formula has much ascorbic acid, and much metaborate for buffering. I decided to eliminate these variables separately. Following PE's rule of "never do one test", I ran two strips with a formula having less ascorbic acid. Here are their graphs:
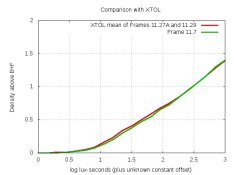 and
and 
Here's the formula. Note the addition of citric acid to maintain pH:
The good news is the right-side rise seems to be gone. But I notice that both graphs have a bit lower EI and a bit higher contrast compared to XTOL and compared to what I was getting with earlier formulas. These differences are probably too small for folks to notice, but I'd like to know why this is happening. I'm mixing propylene glycol into the water to simulate a concentrate, and I'm wondering if PG reduces EI slightly, or if that's due to the very strong buffering, or if the citric acid could do this. So I'll try reducing metaborate and eliminating the citric acid, unless you have a better idea.
Mark Overton
 and
and 
Here's the formula. Note the addition of citric acid to maintain pH:
Sodium sulfite .................. 45 g
Sodium metaborate .......... 5.4 g (very high to give strong buffering)
Ascorbic acid .................. 4.5 g (a normal amount; AA/Phen ratio=90)
Citric acid ....................... 1 g (brings pH back down to 8.16)
Phenidone ...................... 0.05 g (developer)
Propylene glycol ............. 23.5 ml (amount needed in a concentrate for 30 ml/L)
Sodium metaborate .......... 5.4 g (very high to give strong buffering)
Ascorbic acid .................. 4.5 g (a normal amount; AA/Phen ratio=90)
Citric acid ....................... 1 g (brings pH back down to 8.16)
Phenidone ...................... 0.05 g (developer)
Propylene glycol ............. 23.5 ml (amount needed in a concentrate for 30 ml/L)
The good news is the right-side rise seems to be gone. But I notice that both graphs have a bit lower EI and a bit higher contrast compared to XTOL and compared to what I was getting with earlier formulas. These differences are probably too small for folks to notice, but I'd like to know why this is happening. I'm mixing propylene glycol into the water to simulate a concentrate, and I'm wondering if PG reduces EI slightly, or if that's due to the very strong buffering, or if the citric acid could do this. So I'll try reducing metaborate and eliminating the citric acid, unless you have a better idea.
Mark Overton
Again, the graphs show less difference than slight variations in temperature, agitation or other processing parameters will give you. If you want to lower pH without adding a new ion (citrate) to the mix, you could replace some of the sulfite with metabisulfite. Not sure it makes a big difference, but it should reduce complexity. If you reduce amount of metaborate, you also reduce buffer strength, i.e. you turn more knobs at once.The good news is the right-side rise seems to be gone. But I notice that both graphs have a bit lower EI and a bit higher contrast compared to XTOL and compared to what I was getting with earlier formulas. These differences are probably too small for folks to notice, but I'd like to know why this is happening. I'm mixing propylene glycol into the water to simulate a concentrate, and I'm wondering if PG reduces EI slightly, or if that's due to the very strong buffering, or if the citric acid could do this. So I'll try reducing metaborate and eliminating the citric acid, unless you have a better idea.
Again, the graphs show less difference than slight variations in temperature, agitation or other processing parameters will give you. If you want to lower pH without adding a new ion (citrate) to the mix, you could replace some of the sulfite with metabisulfite. Not sure it makes a big difference, but it should reduce complexity. If you reduce amount of metaborate, you also reduce buffer strength, i.e. you turn more knobs at once.
You are correct. But don't forget that I am a perfectionist trying to tame that variability. That approach works well for software I write, but I'm finding that this is a different animal. I have another motive for adding citric acid: chelation. Though PE says there are better chelators out there, citric acid is easy to obtain and will hopefully extend the 45-minute limit after mixing in hard water.
Today, I tried something different: I replaced the metaborate with TEA. The results look great. The density-curve is a nearly perfect match to XTOL:

And the grain and sharpness look the same as well, and JPEG sizes are within 1%:
TEA soup:
 XTOL:
XTOL: 
Here's the formula for the concentrate:
TEA (99%) ...................... 20 ml (22.5 g)
Propylene glycol .............. 5 ml
Ascorbic acid ................... 4.5 g
Citric acid ........................ 4.2 g
Phenidone ...................... 0.05 g
Propylene glycol to make 30 ml
Propylene glycol .............. 5 ml
Ascorbic acid ................... 4.5 g
Citric acid ........................ 4.2 g
Phenidone ...................... 0.05 g
Propylene glycol to make 30 ml
Target pH = 8.16-8.18.
For TMY-2: 11:30 minutes at 20C.
A litre of developer contains 30 ml of concentrate and 45 g of sodium sulfite.
TEA has a number of advantages:
- Stronger buffering due to large quantity (helps with variable sulfite-measurement).
- Concentrate will hopefully not crystallize.
- Phenidone can be replaced with Dimezone S (try 0.1g in the above formula).
- With TEA, pH drops as temperature rises, giving some auto-correction of temperature.
- Avoids borates which damage citrus trees.
- It's harder to locate in some areas.
- 85% TEA is also available, potentially causing inconsistency.
- It's too viscous and must be thinned by another solvent (I used propylene glycol above).
What do you think about all this? And should I go with the metaborate formula in propylene glycol, or this TEA-based formula?
Finally, a question for chemists: Is 4.2 g/L of citric acid enough to chelate all the calcium and magnesium in hard water? If so, that would be another advantage of the TEA-based formula.
Mark Overton
Last edited by a moderator:
Mark;
It is hard to answer your question about chelation as Citric Acid is a poor chelating agent and water contents and quality vary all over the world. Run a keeping test vs Xtol is what I suggest.
PE
It is hard to answer your question about chelation as Citric Acid is a poor chelating agent and water contents and quality vary all over the world. Run a keeping test vs Xtol is what I suggest.
PE
A keeping test will only tell us whether the concentrate keeps, but won't tell us much about the effectiveness of the citric acid chelating agent against hard water. I'm quite confident that citrate won't tie up iron enough to render it ineffective against the Ascorbate.
Here's my take:
Here's my take:
- You can find chelate stability of various citrate salts here.
- The strongest competitors for Ca2+ in any film dev will be sulfite and carbonate. Carbonate also competes for Mg2+.
- With estimates for ion concentration and the numbers taken from the linked pages above you can calculate whether Ca2+ or Mg2+ will likely precipitate.
If you run a keeping test of the dilute working solution, you will see how it performs with your tap water. By having a few volunteers run a similar test, we will learn a lot.
PE
PE
- Joined
- Nov 16, 2004
- Messages
- 3,400
Mark,
I don't know of any developer produced on a non-profit basis that contains a chelating agent to prevent hard water cloudiness.This includes all Crawley's published formulae, PMK Pyro,the Pyrocats. Gainer's PC-TEA etc.I have not come across any grumbles on the internet about cloudiness produced by these developers.My guess is that manufacturers include a chelating agent because it is easier to sell a developer that produces a clear solution in hard water.So IMO chelating agent is interesting but not necessary in a non-profit developer.
I once wrote to Geoffrey Crawley re glycol in developers and he replied IIRC that he put some in his commercial developers to help prevent freezing in winter,evidently he considered glycol not harmful to the developing properties.
Re metaborate and TEA , IMO it would be interesting if a recommended non-crystallising metaborate formula could be found for those who only have metaborate not TEA.But at this time your work does seem to suggest that TEA might turn out to give a result closer to Xtol if this line is pursued.
I don't know of any developer produced on a non-profit basis that contains a chelating agent to prevent hard water cloudiness.This includes all Crawley's published formulae, PMK Pyro,the Pyrocats. Gainer's PC-TEA etc.I have not come across any grumbles on the internet about cloudiness produced by these developers.My guess is that manufacturers include a chelating agent because it is easier to sell a developer that produces a clear solution in hard water.So IMO chelating agent is interesting but not necessary in a non-profit developer.
I once wrote to Geoffrey Crawley re glycol in developers and he replied IIRC that he put some in his commercial developers to help prevent freezing in winter,evidently he considered glycol not harmful to the developing properties.
Re metaborate and TEA , IMO it would be interesting if a recommended non-crystallising metaborate formula could be found for those who only have metaborate not TEA.But at this time your work does seem to suggest that TEA might turn out to give a result closer to Xtol if this line is pursued.
If you run a keeping test of the dilute working solution, you will see how it performs with your tap water. By having a few volunteers run a similar test, we will learn a lot. PE
I'll need to do that. My intention is to make the dev last more than 45 minutes with hard water before it gets cloudy. It would be nice if it could sit around for a few hours or a day. The wikipedia article on citric acid here says it's an "excellent" chelating agent. But PE says there are much better choices around (Kodak likes DTPA). I guess we don't know if citric acid will chelate well in a developer, so as PE says, tests must be run. Also, I understand that the Fenton reaction with iron will eventually destroy the ascorbate, but that takes days or weeks, and this is a one-shot dev, so I don't care about Mr. Fenton. Although if I can chelate the iron for free, I will.
Rudi, thanks for the link to the list of stability constants. There's much useful data in there. I just looked up Glycine and decided it would be pretty weak.
Mark Overton
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |


 I caught the tear-down:
I caught the tear-down: