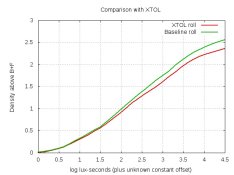
Crystallization
I've been experimenting with more concentrates using propylene glycol as the solvent, and characterizing the issue of crystallization. By way of review:
* Paul Verizzo and I independently discovered that sodium metaborate dissolves in propylene glycol (PG).
* I discovered that having some metaborate dissolved in PG increases the solubility-limit of ascorbic acid.
* And I discovered that if one isn't careful, the solution of PG+metaborate+ascorbic can crystallize, causing the solution to separate into a cream and a liquid. It starts as smoke-like wisps, or cloudiness, or a few crystals appearing on the bottom. This can happen while mixing the concentrate hot, or it can start after a few days of sitting at room-temperature.
Here's a smattering of observations about crystallization:
1. It is not solely or even primarily a function of concentration-ratio (weight of powders in grams divided by final volume in ml). One solution with a ratio of 0.33 failed, and another with a ratio of 0.40 succeeded.
2. All solutions with a metaborate-ratio (weight of metaborate in grams divided by final volume in ml) exceeding 0.12 failed. None with less than 0.12 failed. Of two with ratios of exactly 0.12, one passed and one failed.
3. All solutions with a metaborate/ascorbic ratio of at least 0.533 failed. This ratio determines the alkalinity of the concentrate, and it appears that it dislikes alkalinity.
4. Phenidone and perhaps especially Dimezone S make the crystallization problem worse.
5. Higher temperatures while mixing ascorbic acid and Phenidone encourage crystallization during the mixing process.
6. Metaborate alone in PG will not crystallize. You need ascorbic acid in there.
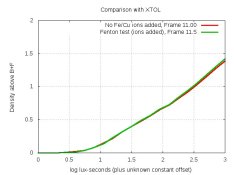
That's what I know. Numbers 2 and 3 encourage one to reduce the metaborate, but if you do, you can't dissolve all the ascorbic acid in a reasonable time (or at all?). So there's a trade-off. In fact, it seems that there's a narrow zone where one can dissolve all the ascorbic acid while avoiding crystallization. And the developer D316 that I created back in January just happened to hit that zone. Alan Johnson, who named that developer after its posting-number, mixed the concentrate back then and has been testing it. I have a similar developer that's been sitting since then as a longevity-test. D316 is:
Propylene glycol .................... 16 ml (hot)
Sodium metaborate 4 mol ..... 2.2 g
Ascorbic acid ......................... 4.5 g
Phenidone ............................. 0.05 g
The result is almost exactly 20 ml of concentrate.
A litre of developer contains 20 ml of concentrate and 45 grams of sodium sulfite (but I'm thinking of boosting the sulfite).
I tried changing the amount of metaborate. At 2.0 g, I could not dissolve all the ascorbic acid. At 2.4 g, the solution crystallized while hot. That tells you how narrow the workable zone is. Also, D316 showed the beginning of crystallization when I mixed it too hot (around 90C), consistent with observation 5. Alan mixed his at 70C with no problem.
I posted this information so other home-brewers won't have to re-discover these things. I hope it helps somebody.
Mark Overton