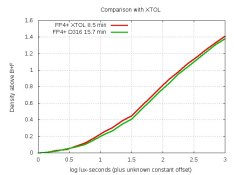
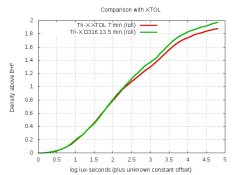
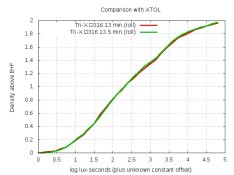
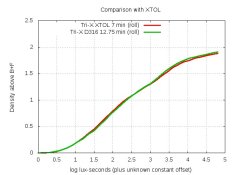
AAAAAAAAAAAAAAA! I scream every time someone asks about % which is about once a month.
There is weight / weight, weight / volume, volume / weight, volume / volume and thus you see how solutions are "written up" in the chemical literature when % is to be expressed.
True % is, for a 10% solution of a solid in water, 10 g of solid and water to 100 ml for wt/vol, or 10 g of solid and 90 g of water for wt/wt.
It goes on. All of these different methods exist for handy use of viscous or organic solutions, especially in the dark.
PE
There is weight / weight, weight / volume, volume / weight, volume / volume and thus you see how solutions are "written up" in the chemical literature when % is to be expressed.
True % is, for a 10% solution of a solid in water, 10 g of solid and water to 100 ml for wt/vol, or 10 g of solid and 90 g of water for wt/wt.
It goes on. All of these different methods exist for handy use of viscous or organic solutions, especially in the dark.
PE