I remember reading of some exposed photographic plates that were discovered from a failed polar expedition. The plates were over 80 years old at the time of discovery yet produced usable images.
Okay folks, I cut the roll into strips and froze them. Each strip is in a plastic film-can, and they're all in a metal cooking pot with a snugly fitting lid, providing two layers of shielding against light. Thanks for the ideas about this; ageing film is a variable that I don't need.
Here's an update of the baseline developer I posted a couple weeks ago. The new one litre formula:
Sodium sulfite ..................... 45 g
Sodium metaborate ............ 2.6 g
Ascorbic acid ...................... 4.5 g
Phenidone .......................... 0.05 g (I use a 2% solution of propylene glycol)
Target pH is 8.15. For TMY2: 12.25 minutes at 20C.
The only difference between this and the baseline dev of two weeks ago is metaborate went from 2.0 g to 2.6g, raising the pH to be closer to what XTOL uses. With the higher pH, there will be less risk of failure with films that dislike low pH.
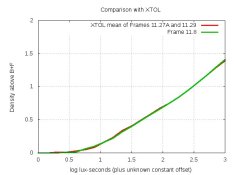
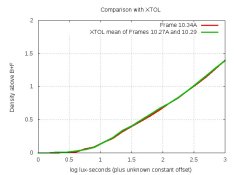
Here are the curves for this developer and XTOL:
And here are crops of neg-scans:
XTOL:

This dev:

The grain looks a bit finer in loupes, and the JPEG sizes are 1% smaller. But XTOL looks a hair sharper to me in those crops.
This dev is merely a stepping-stone. Things I plan to try are (1) reduce both metaborate and ascorbic acid to reduce concentration-ratio, (2) increase pH a little more to match XTOL, and (3) replace metaborate with TEA and replace Phenidone with Dimezone S to see if a concentrate based on TEA and Dimezone S will crystallize.
Mark Overton
EDIT: BTW, I didn't mean for this posting to sound closed to inputs from others. If you have any comments or ideas to try, I'd like to hear them.



 But I figured out a trick for getting a temperature into the meter: apply a resistance to the ATC connector using a potentiometer. That way one can simply dial-in the temp seen on the thermometer. This evening, I tried various resistors and got a table of corresponding temperatures from the meter, so now I know what kind of resistor-network to solder together. That's another electronic hack until I find a temp probe (and I like your idea of an electrode with built-in temp sensor).
But I figured out a trick for getting a temperature into the meter: apply a resistance to the ATC connector using a potentiometer. That way one can simply dial-in the temp seen on the thermometer. This evening, I tried various resistors and got a table of corresponding temperatures from the meter, so now I know what kind of resistor-network to solder together. That's another electronic hack until I find a temp probe (and I like your idea of an electrode with built-in temp sensor).