NP, just clarifying my comment combined with yours.
PE
PE
Home made pH meter? Sounds pretty good for home made.
Also, buffers, for whatever reason, often are off a little bit from calculated values. You can made fine adjustments to them. For borate buffers, use boric acid to make it more acidic, and sodium hydroxide to raise the pH.
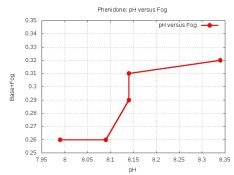
I have never heard anyone other than yourself say that Phenidone produces more fog than Dimezone-S.
Mark;
Another good reason to use Dimezone-S, unless there is an antifoggant in XTOL!
PE
Way back when, he said he was going to get a patent on his special chelating agent, but I have seen nothing in the literature yet. PE
Shaking entrains more oxygen. PE
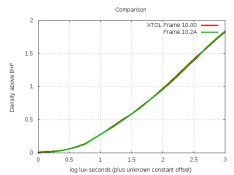
Mark,I mixed a new batch of XTOL a couple of days ago, storing it in five 1-litre bottles. And I thought of a way of making it last longer: Store the extra bottles in the refrigerator. Sulfite will precipitate out of solution, but when it's time to start a new bottle, warming and shaking it should bring the precipitate back into solution. Kirk Keys reminded me that chemical reactions proceed half as fast with each 10C temperature-drop, so refrigeration should make XTOL last around 1.5-2 years instead of 6 months. Is there any reason why this would not work?
Mark Overton
 -
- 
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |