albada
Subscriber
You might not be as far out as you think, the patent for XTOL lists the final pH as 8.20+/-0.05 which takes into account all the variations in the ingredients and the mixing process they considered at the time. You have done very well to get so close and it may not make any practical difference to the grain, sharpness or the characteristic curve.
Peter, your posting prodded me to do the experiment below this evening, because it reminded me that I'm halfway through figuring out an exact clone of the XTOL that's now in production.
One difficulty is ascorbate is hard to find, so to clone XTOL, one must separately convert some ascorbic acid into ascorbate by adding sodium bicarbonate (baking soda). The problem with that is if the correct amounts of both ingredients are mixed, it will theoretically take a nearly infinite amount of time to convert all the ascorbic acid. To see why, suppose there is one molecule remaining of each ingredient. It will require perhaps years of stirring before those two molecules touch each other. So to complete the conversion in reasonable time, an excess of bicarbonate is needed. But what will that do to the developer?
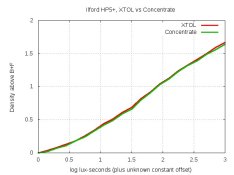
So I developed one test-strip in XTOL, and a second in XTOL with 0.5 g/L of bicarbonate added. Their characteristic curves are virtually identical, and grain looks the same (but I didn't check sharpness). It appears that a small amount of bicarbonate won't hurt an XTOL-like developer, so it's feasible for home-brewers to create ascorbate this way. But I need to try the dev with and without DTPA to see if that makes any difference. Mytol dropped it, so I doubt it helps image-quality any.
Mark Overton