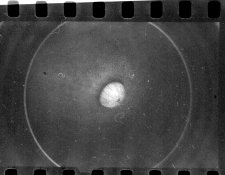
here is one of the results from last night's run:
two strips of tri-x in:
500ml water, 3 tbsp dextrose, 3tbsp, sodium carbonate, 1 tbsp sodium bicarbonate, 0.3 grams KBr, 72F
dextrose & sodium carbonate are heated together in the microwave for over an hour to generate the developing agents....glucic acid...and perhaps others ( gluconic acid is a new suspect ).....then the other ingredients are added...I thought the sodium bicarbonate was going to create a buffer solution and lower the pH...but pH was 11 before & after I added it....KBr was guesstimated at 0.3 grams because in a previous test 1.1 grams was helpful but waayyyy too much
one strip of tri-x was removed after 45 minutes, the other after 90 minutes
both negatives had decent images without all the fog I used to get....after only 45 minutes the negative looked usable, but thin...the negative that was developed for 90 minutes looked pretty good
here's a scan of one of the 90min images:

Today's experiment is heating table sugar aka sucrose with vinegar for several hours. Sucrose is made from glucose ( aka dextrose ) joined to fructose. Heating with vinegar/acetic acid is supposed to split the sucrose into glucose & fructose. After I treat the sucrose with the acetic acid I will then react it with the sodium carbonate the usual way and see if it works. I tried sucrose before & it did not work the way honey ( contains glucose & fructose ) and glucose did.
N.B. to be clear....dextrose is "right-handed" glucose...so all dextrose is glucose, but not all glucose is dextrose...but there apparently isn't much left-handed glucose lying around...so both names tend to be used. Sorry if it gets confusing when I use different names for the same thing.
I read that fructose breaks down even easier than glucose/dextrose...so I ordered some fructose to test that. If both glucose and fructose generate developers that would make honey an even more convenient raw material since it contains both.
I thought this was funny...while looking for sources of fructose online I ended up at several bodybuilding websites...they seem to have the best prices for dextrose & fructose ...well in their explanations of the benefits of dextrose the bodybuilding websites explained that dextrose dissolves easily in water...and after using the word "dissolved" in parentheses it said "disappears" --- as if Bruno the weightlifter might not know what "dissolve" means.