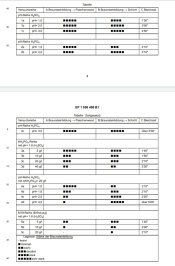
Yes ,,I added sodium bisulfate, not sulfate. This is a substitute for sulfuric acid and works fine. Instead of using H2SO4, I used NaHSO4. This might also reduce emulsion softening from permanganate a bit, but I can't be sure about it, I haven't made any side by side tests.
I understand your smart trick,
But it won't work, I think in the long run,
Sulfuric acid is essential and indispensable, and you cannot add any ingredient to replace it.
- I only read it five minutes ago, a post written by the great man Ron Murray, who said that without sulfuric acid you wouldn't get a good reverse image.
It was easier for him to say that he used sodium bisulfate and get rid of sulfuric acid.
I know you are afraid of sulfuric acid, but it is (an evil that must exist) in that formula.



 I only staring to mix my own developers in1976/7 and the first two bottles Ilford batch code showed it was made in 1961, and it's still OK today although it's a couple of years since I used some as a test - I've used a lot more batches since those first two 25gm bottles.
I only staring to mix my own developers in1976/7 and the first two bottles Ilford batch code showed it was made in 1961, and it's still OK today although it's a couple of years since I used some as a test - I've used a lot more batches since those first two 25gm bottles.