-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Superadditivity in developers - and beyond
-
A
- Thread starter Photo Engineer
- Start date
Recent Classifieds
-
For Sale Kodak 2465 microfilm
- Started by MCB18
-
Want to Buy Linhof Technika 4x5 240 cam.
- Started by Logical1
-
Free Free Darkroom Stuff
- Started by Reinhold
-
For Sale Zeiss Distagon 35mm f2 ZF Industrial
- Started by Jammoh
-
For Sale FS: Fujinon 250mm f/6.7 Lens
- Started by B.S.Kumar
Forum statistics
Patrick;
Generally, any reaction product in chemistry will retard any further reaction to some extent. So, the graph is perfectly reasonable. That is what happens with oxidized HQ which turns into HQ-mono sulfonate. The quinone vanishes from the equation and therefore the reaction speeds up.
The conversion of oxidized ascorbic acid back to ascorbic acid is through an electron transfer that oxidizes the sulfite to sulfate. These two types of reaction are not identical and yet do involve oxidation and reduction reactions.
PE
Generally, any reaction product in chemistry will retard any further reaction to some extent. So, the graph is perfectly reasonable. That is what happens with oxidized HQ which turns into HQ-mono sulfonate. The quinone vanishes from the equation and therefore the reaction speeds up.
The conversion of oxidized ascorbic acid back to ascorbic acid is through an electron transfer that oxidizes the sulfite to sulfate. These two types of reaction are not identical and yet do involve oxidation and reduction reactions.
PE
- Joined
- Sep 20, 2002
- Messages
- 3,699
Patrick;
Generally, any reaction product in chemistry will retard any further reaction to some extent. So, the graph is perfectly reasonable. That is what happens with oxidized HQ which turns into HQ-mono sulfonate. The quinone vanishes from the equation and therefore the reaction speeds up.
PE
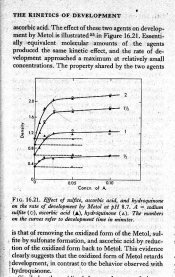
I think you misread the graph. Hydroquinone, sulfite and ascorbic acid are treated separately. There is no effect of hydroquinone on the development by metol at any concentration of hydroquinone that was tested, as you can see by the fact that the empty triangle symbols fall on a straight horizontal line originating at the 0 concentration point. The development by metol is accelerated by either ascorbate or sulfite.
The reaction products of hydroquinone accelerate development by hydroquinone, according to the same reference. Ascorbic acid "tames" hydroquinone.
I did what I promised. I mixed a solution of borax, metol and hydroquinone. A snip test of HP5+ with this solution yielded a density above base of 1.3 after 2 minutes at 70 F. I added 3.5 grams of sodium sulfite (anh) to this solution and got a snip test density of 3.04. I added the molar equivalent of the sulfite, 4.9 g, of ascorbic acid converted to sodium ascorbate by 2.45 g of NaHCO3 in a small amount of water. I let the effervescence subside before adding it to the other ingredients. The snip test from this solution measured 3.03 density. I then prepared a solution of metol, borax and sodium ascorbate in an amount equivalent to 6 g of the acid, with the same amounts of metol and borax as the original solution but no hydroquinone. The snip test measured 3.02 density.
I developed a strip of pictorial negatives, all taken from the same roll of the same scene, in each of the three solutions for 8 minutes at 70F. There were slight differences in contrast, about as one might expect from the differences in snip test density. The grain in 10X enlargement is quite good.
I will give a more complete report when I can get around to scanning the prints from each concoction.
Cheers, Y'all.
Patrick;
It explicitly states that the oxidation products retard development, and their removal accelerates development rate. Therefore, they state that sulfite addition accelerates HQ and Metol development. That is what I was referring to as well as the reference to the superadditive effect between Metol and HQ. The utility of the Elon Ascorbic acid developer (Metol=Elon), known at Kodak as EAA is well know as well.
PE
It explicitly states that the oxidation products retard development, and their removal accelerates development rate. Therefore, they state that sulfite addition accelerates HQ and Metol development. That is what I was referring to as well as the reference to the superadditive effect between Metol and HQ. The utility of the Elon Ascorbic acid developer (Metol=Elon), known at Kodak as EAA is well know as well.
PE
- Joined
- Sep 20, 2002
- Messages
- 3,699
"Therefore, they state that sulfite addition accelerates HQ and Metol development."
I saw no such statement. I saw no place where they even implied such a statement. They did no tests that could be interpreted in that way. Those who produced the graph I sent did not present any evidence that HQ adds to the development by Metol, either synergistacally or simply. They did present evidence that sulfite accelerates development by Metol without HQ. I see no place where these researchers stated that sulfite accelerates HQ and Metol development.
The graph clearly shows that there is not even an additive let alone superadditive effect between Elon and hydroquinone without the presence of either a sulfite or ascorbic acid. The activity of Elon is approximately doubled by the addition of either sulfite or ascorbate.
My own experiments, which anyone can repeat, show that either sulfite or ascorbate at the same molar concentration stimulate the same degree of superadditivity between Metol and HQ, and at the same pH. The pH of a 10 g/l borax solution is bountifully sufficient for either an MQS or an MQC developer. The molar concentration of S need only be the same as that of the C. If you do not want stain, you can substitute as much sodium ascorbate as you want for the Q.
At any rate, I learned what I set out to learn. Ascorbic acid, which is soluble in glycol or TEA, can be substituted for sulfite in such developers as the Pyrocat series and also in PMK in quite small amounts. There is no loss of activity if, OTH, sodium ascorbate is substituted for hydroquinone in the usual MQ or PQ developers.
I doubt that Kodak had much interest in creating or promoting staining developers in glycol solution.
I saw no such statement. I saw no place where they even implied such a statement. They did no tests that could be interpreted in that way. Those who produced the graph I sent did not present any evidence that HQ adds to the development by Metol, either synergistacally or simply. They did present evidence that sulfite accelerates development by Metol without HQ. I see no place where these researchers stated that sulfite accelerates HQ and Metol development.
The graph clearly shows that there is not even an additive let alone superadditive effect between Elon and hydroquinone without the presence of either a sulfite or ascorbic acid. The activity of Elon is approximately doubled by the addition of either sulfite or ascorbate.
My own experiments, which anyone can repeat, show that either sulfite or ascorbate at the same molar concentration stimulate the same degree of superadditivity between Metol and HQ, and at the same pH. The pH of a 10 g/l borax solution is bountifully sufficient for either an MQS or an MQC developer. The molar concentration of S need only be the same as that of the C. If you do not want stain, you can substitute as much sodium ascorbate as you want for the Q.
At any rate, I learned what I set out to learn. Ascorbic acid, which is soluble in glycol or TEA, can be substituted for sulfite in such developers as the Pyrocat series and also in PMK in quite small amounts. There is no loss of activity if, OTH, sodium ascorbate is substituted for hydroquinone in the usual MQ or PQ developers.
I doubt that Kodak had much interest in creating or promoting staining developers in glycol solution.
Patrick, the comment I reference is at the bottom of Page 366, right column which describes the experiment in figure 16.21 in detail. "oxidation products.... retard development" and then "activity returned by addition of sodium sulfte".
This is the problem with a non chemist reading an argument on chemical reactions. This is A + B = C where C buildup retards development.
If C has nowhere to go the reaction can virtually cease, but if C + D = something else, the reaction speeds up.
Now, lets write Silver halide + HQ = Quinone + Silver metal. Silver metal is a solid, but quinone is in solution and blocks up the furhther reaction of HQ, all by itself or by making Quinhydrone.
If you have Q + sulfite = HQ monosulfonate, then the reaction speeds back up again. QED.
PE
This is the problem with a non chemist reading an argument on chemical reactions. This is A + B = C where C buildup retards development.
If C has nowhere to go the reaction can virtually cease, but if C + D = something else, the reaction speeds up.
Now, lets write Silver halide + HQ = Quinone + Silver metal. Silver metal is a solid, but quinone is in solution and blocks up the furhther reaction of HQ, all by itself or by making Quinhydrone.
If you have Q + sulfite = HQ monosulfonate, then the reaction speeds back up again. QED.
PE
- Joined
- Sep 20, 2002
- Messages
- 3,699
The problem I have is that your QED doesn't follow from the experiment, however true it may be in general. The speeding up shown on the graph is of Metol without hydroquinone. Metol with hydroquinone but without sulfite shows no speeding up. Metol with either sulfite or ascorbic acid shows speeding up due to the formation of a Metol sulfonate by sulfite or to the reduction of the oxidized form back to Metol. Hydroquinone was not present at the same time as either sulfite or ascorbic acid. Therefore, any speeding up of the development in these experiments could not have come from formation of hydroquinine monosulfonate. I quote from the conclusion drawn from the experiment. "This evidence clearly suggests that the oxidized form of Metol retards development, in contrast to the behavior observed with hydroquinone." On page 366 you see the evidence that the oxidation products of hydroquinone clearly accelerate development and that the addition of sulfite retards development by the hydroquinone solution.
I would have been a chemist if I had not switched from chemical engineering to aeronautical after three years. Beside, logic is the same for all who learn to use it. Logically, you should have seen that the experiment dealt with only the one agent. The usually quoted theory that hydroquinone regenerates the Metol in MQ synergism does not apply unless sulfite or ascorbate is present. It certainly does not apply when hydroquinone is not present.
I would have been a chemist if I had not switched from chemical engineering to aeronautical after three years. Beside, logic is the same for all who learn to use it. Logically, you should have seen that the experiment dealt with only the one agent. The usually quoted theory that hydroquinone regenerates the Metol in MQ synergism does not apply unless sulfite or ascorbate is present. It certainly does not apply when hydroquinone is not present.
The experiment on the precending page plus the other data show that the oxidation product of metol inhibits further development. Cross oxidation to remove the oxidation product of Metol speeds up the reaction. Either sulfite or ascorbate can act in this capacity.
This is stated clearly in that paragraph. (At least to a chemist). Sorry Patrick, but that is my professional read on it. You are misinterpreting the data.
PE
This is stated clearly in that paragraph. (At least to a chemist). Sorry Patrick, but that is my professional read on it. You are misinterpreting the data.
PE
- Joined
- Sep 20, 2002
- Messages
- 3,699
Maybe I didn't make my interpretation clear. Your statements until this one have implied that hydroquinone's reaction products are what make the MQ synergism work. The experiment in question does not investigate that synergism in the presence of sulfite. In fact, hydroquinone has practically no reaction at pH 8.7. What reaction it has at high pH is accelerated by its own reaction products when sulfite is not present. Addition of sulfite retards development by hydroquinone. OTH, Metol is capable of development without sulfite, but development is retarded by its own reaction products. The explanation for the accelerating effect of sulfite added to a Metol developer was the formation of a Metol sulfonate, not a hydroquinone monosulfonate. Ascorbic acid had the same effect, mole for mole, but by a different mechanism. Hydroquinone, I repeat, had no bearing on the experiments because it was not present in any test where sulfite or ascorbate was present. If hydroquinone had been present, the results could not hav unequivocally supported the conclusion that was drawn, that the reaction products of Metol, in contrast to hydroquinone, retarded development by Metol.
Why are we arguing about these facts? How did I misinterpret the data? What difference does it make if I did? I got a working hypothesis out of it that did work as expected.
All I can say is I'm glad you are not my physician. If you don't take this personally, I won't take your slurs on my logical thinking personally. You are the first person, other than my own father, who ever accused me of not thinking straight. I was about 6 years old at the time, and he was a Professor at St.Louis University. A close friend of his, and my sponsor at Confirmation, was Dr. Vernon J, Bourke, a noted Thomistic Philosopher.
Why are we arguing about these facts? How did I misinterpret the data? What difference does it make if I did? I got a working hypothesis out of it that did work as expected.
All I can say is I'm glad you are not my physician. If you don't take this personally, I won't take your slurs on my logical thinking personally. You are the first person, other than my own father, who ever accused me of not thinking straight. I was about 6 years old at the time, and he was a Professor at St.Louis University. A close friend of his, and my sponsor at Confirmation, was Dr. Vernon J, Bourke, a noted Thomistic Philosopher.
- Joined
- Sep 20, 2002
- Messages
- 3,699
Sorry about that. I think you think I'm trying to theorize about synergism. (We used that term at NASA as applied to human endeavors. Sometimes two persons can do more work together than the sum of what the two can do seperately.) All I wanted to know at first was whether it was reasonable to expect ascorbic acid to do the same job of promoting synergism between developing agents. The graph I sent did not satisfy me because it only dealt with one agent, considering that ascorbate is not much of an agent at pH 8.7
but neither is hydroquinone. I have the answer. Thanks.
but neither is hydroquinone. I have the answer. Thanks.
- Joined
- Dec 5, 2004
- Messages
- 64
- Format
- ULarge Format
This discussion certainly makes me want to test a few of these compounds in my current developer. Photographer's Formulary sells Urea; any suggestion as to what amount to add to a liter of film developer as a start? They also sell ethylene diamine; here again any suggestions about starting quantity, and what effect on the film one might expect?
Patrick, your observations about the reaction are correct. The interpretation of the data is due to Kinetics and the nature of chemical equillibria, no more.
Sulfite is NOT the cause of the synergism between HQ and Metol. As M&J says in the text, the rection of each is accelerated (they specifically refer to a Metol experiment as their major talking point).
So, the bottom line is that what you say will work, but the reasoning is different to a chemist as to why. Thats all. Sorry if my comments seemed otherwise to yours.
Superadditivity is an older word for synergy in some cases. It is still used in the MQ case.
John;
Urea is hit and miss. Remember, it softens film so be careful. I would start at 1 g/l and go as high as perhaps 20. Remember that it can cause reticulation. Ethylendiamine sulfate will cause fog if misused. I would use no more than about 10 g/l and that is it. It will change the pH too, IIRC.
I am only bringing this up for experimenters to tinker with, so I'll have to say good luck. It is a chancy thing with both and that is why they are not used in production chemistry AFAIK.
PE
Sulfite is NOT the cause of the synergism between HQ and Metol. As M&J says in the text, the rection of each is accelerated (they specifically refer to a Metol experiment as their major talking point).
So, the bottom line is that what you say will work, but the reasoning is different to a chemist as to why. Thats all. Sorry if my comments seemed otherwise to yours.
Superadditivity is an older word for synergy in some cases. It is still used in the MQ case.
John;
Urea is hit and miss. Remember, it softens film so be careful. I would start at 1 g/l and go as high as perhaps 20. Remember that it can cause reticulation. Ethylendiamine sulfate will cause fog if misused. I would use no more than about 10 g/l and that is it. It will change the pH too, IIRC.
I am only bringing this up for experimenters to tinker with, so I'll have to say good luck. It is a chancy thing with both and that is why they are not used in production chemistry AFAIK.
PE
- Joined
- Oct 11, 2006
- Messages
- 2,195
- Format
- Multi Format
Patrick;
Sulfite also drags the oxidation of HQ to the right by scavenging the quinone by generating HQ monosulfonate which itself is a weak developer.
But, as a number of people have said, the use of more than two developing agents is a real reach. I know that Haist has said that, and probably Henn and Lee as well.
BTW, I have also made mention elsewhere of ETA developers. They are distinguished from superadditive developer combinations by having the pH adjusted to be optimum for the primary developer. The primary developer is present at very low concentrations. The other developer is not really active under these conditions except to regenerate the primary developer. These are unique and AFAIK there are not currently on the market.
PE
I am a chemist by training, though not an expert in photographic chemistry. However, I was reading about properties of developers one day and it occurred to me that an ascorbate/para aminophenol developer adjusted to pH 9 or so should activate the para aminophenol (perhaps weakly) but not the ascorbate, and that the ascorbate should regenerate the para aminophenol under these conditions. Does this sound something like what you are describing?
One more thing. I understand that when ascorbate is oxidized it increases the acidity of the solution. If so would a developer such as I outlined above have a compensating effect by lowering the local pH in the gel in the areas of most active development, thereby inhibiting development in those areas?
Alan
- Joined
- Sep 20, 2002
- Messages
- 3,699
It certainly appears to work with phenidone and ascorbate in PC-TEA. At 1+50 dilution there will be almost 20 ml of TEA, which is about 17.8 grams, in a liter of working solution. There will be about 1.8 grams of ascorbic acid, which can make some kind of salt with about 1.5 grams of the TEA. With the 99% grade of TEA, the chart in the Dow Chemical manual for ethanolamines shows a pH of about 8.8 for the working solution. I have ordered some pH test strips that should help me be a little more specific as to pH in a week or so. This working solution is as active as D-23 full strength. P-aminophenol may require somewhat higher pH, but it should be in the range provided by some amount of borax. P-aminophenol and ascorbic acid are both soluble in propylene glycol or TEA. Higher pH could be obtained by using diethanolamine, but I have not tried it.
In experiments I did today, the results from HP5+ developed in PC-TEA for 8 minutes at 70 F are no more grainy than those from D-23 full strength. This is hard to show in scanned images. There, the only difference I can see is a slightly greater sharpness in fine detail in the PC-TEA, but file size limitations here would make that hard to present.
In experiments I did today, the results from HP5+ developed in PC-TEA for 8 minutes at 70 F are no more grainy than those from D-23 full strength. This is hard to show in scanned images. There, the only difference I can see is a slightly greater sharpness in fine detail in the PC-TEA, but file size limitations here would make that hard to present.
I am a chemist by training, though not an expert in photographic chemistry. However, I was reading about properties of developers one day and it occurred to me that an ascorbate/para aminophenol developer adjusted to pH 9 or so should activate the para aminophenol (perhaps weakly) but not the ascorbate, and that the ascorbate should regenerate the para aminophenol under these conditions. Does this sound something like what you are describing?
One more thing. I understand that when ascorbate is oxidized it increases the acidity of the solution. If so would a developer such as I outlined above have a compensating effect by lowering the local pH in the gel in the areas of most active development, thereby inhibiting development in those areas?
Alan
Alan;
Yes, this might work. It is not super additivity but rather probably fits into the realm of the ET (Electron Transport) developer mechanisms. However, if the pH drops then the activity could fall off overall.
PE
- Joined
- Sep 20, 2002
- Messages
- 3,699
There can be no doubt that the phenidone-ascorbate pair is synergistic at pH 8.8 without sulfite. How does one differentiate between the synergism of electron transfer and that of superadditivity? What if I added sulfite to the PC-TEA working solution. Would I expect to find an increase in activity even if I kept pH constant? So far as I know, there is no ascorbic compound equivalent to hydroquinone monosulfonate. I am asking questions, not being derisive. I think one could make a PC-TEA-sulfite working solution, test it, and then make a PC-TEA solution with the same pH by adding a little TEA to the working solution without sulfite. Concentrations of P, C and the pH would be the same in both, the only difference being some amount of sulfite.
You can demonstrate ET effectively by using a very very tiny quantity of one (the most active developer) and a very large amount of the less active or inactive developing agent.
One is quite active by itself, but in too low of a quantity to do more than just barely work and the other does virtually nothing by itself.
This argument is only true at the chosen pH.
That is one method of testing.
It is hard to differentiate between synergy and ET development. In synergy both developers are used at a relatively similar concentration and could do the job alone, so for example many developers with HQ and Metol can be reformulated to use just Metol at the right concentration. And they use each at about the same amount.
PE
One is quite active by itself, but in too low of a quantity to do more than just barely work and the other does virtually nothing by itself.
This argument is only true at the chosen pH.
That is one method of testing.
It is hard to differentiate between synergy and ET development. In synergy both developers are used at a relatively similar concentration and could do the job alone, so for example many developers with HQ and Metol can be reformulated to use just Metol at the right concentration. And they use each at about the same amount.
PE
- Joined
- Nov 16, 2004
- Messages
- 3,374
For the PC-TEA-sulfite system I developed Delta 100 in PC-TEA 1:100 with added sulfite 0,10,30,70 g/L 17.5min 68F as noted in a previous thread.Sulfite slightly raised the pH to 9.5,but if there was any superadditive effect it was not detected as all the negatives taken at the same EI have the same density by visual observation.
Interesting experiment Alan.
I might add to my previous post that one of the ingredients in an ET developer need not even be a developer. And, actually that was the case in Kodak's first use of this type of development. One reagent did not develop silver under any conditions, but very good development took place when a tiny tiny amount of a developing agent was added at the right pH.
PE
I might add to my previous post that one of the ingredients in an ET developer need not even be a developer. And, actually that was the case in Kodak's first use of this type of development. One reagent did not develop silver under any conditions, but very good development took place when a tiny tiny amount of a developing agent was added at the right pH.
PE
- Joined
- Nov 16, 2004
- Messages
- 3,374
G.W.Crawley investigated the effect of minimal sulfite, about 0-5 g/L, in sulfite metol system on his way to FX-1. The minimal sulfite made a lot of difference here.I wonder if minimal ascorbate has similar effect with metol.
Alan;
This is the gist of what Mees and James report from experiments many many years ago and what Patrick has excerpted the figure from. They found that any sulfite in the presence of Metol or HQ will speed up their individual reactions due to the fact that Sulfite reacts with the oxidized forms of these developers.
On top of that HQ and Metol are superadditive, and so you have a multiplicity of effects going on.
PE
This is the gist of what Mees and James report from experiments many many years ago and what Patrick has excerpted the figure from. They found that any sulfite in the presence of Metol or HQ will speed up their individual reactions due to the fact that Sulfite reacts with the oxidized forms of these developers.
On top of that HQ and Metol are superadditive, and so you have a multiplicity of effects going on.
PE
- Joined
- Sep 20, 2002
- Messages
- 3,699
But Metol and hydroquinone are not superadditive without sulfite. That also is shown in the graph in Mees and James.
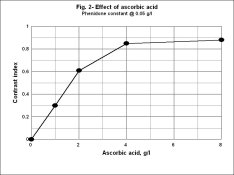
The ratio of ascorbic acid to Phenidone in PC-TEA is about 40 to 1 by weight. I don't know the molecular weight of Phenidone but that of ascorbic acid is 176. That was the ratio at which the increase in activity levelled off as ascorbic acid was added to 0.05 moles/liter of phenidone. I dont remember where I got the molecular weight of Phenidone for that experiment, nor what value I used. I am looking at the graph. I will look up the text. I'm sure you know the MW.
The ratio of ascorbic acid to Phenidone in PC-TEA is about 40 to 1 by weight. I don't know the molecular weight of Phenidone but that of ascorbic acid is 176. That was the ratio at which the increase in activity levelled off as ascorbic acid was added to 0.05 moles/liter of phenidone. I dont remember where I got the molecular weight of Phenidone for that experiment, nor what value I used. I am looking at the graph. I will look up the text. I'm sure you know the MW.
Attachments
- Joined
- Nov 16, 2004
- Messages
- 3,374
I mixed an ascorbate clone of FX-1 where sodium sulfite was replaced by sodium ascorbate: Metol 0.5g ,Sodium Ascorbate 5g ,Sodium Carbonate 2.5g ,water to 1L ,pH about 11. Delta 100 was developed in this 18m 68F. It gave somewhat overdeveloped negs with a high EI.
It is known from Crawley's experiments that if there is no sulfite the action with metol and carbonate alone is very slow.Therefore it follows that both the sulfite in FX-1 and the ascorbate in the clone must be participating in the development,but if by chemically similar reactions seems not to be known.
BTW this clone of FX-1 is similar to a developer listed by Patrick Gainer in his article "Vitamin C developers" at Unblinkingeye.com.
It is known from Crawley's experiments that if there is no sulfite the action with metol and carbonate alone is very slow.Therefore it follows that both the sulfite in FX-1 and the ascorbate in the clone must be participating in the development,but if by chemically similar reactions seems not to be known.
BTW this clone of FX-1 is similar to a developer listed by Patrick Gainer in his article "Vitamin C developers" at Unblinkingeye.com.
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |