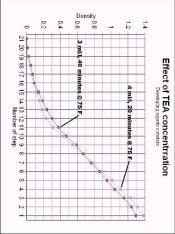
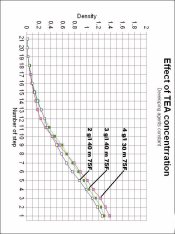
Ethyl ascorbate is what's called an ester. To make an ester you start with an alcohol and an carboxylic acid in a mineral acid solution. The two combine to form and ester. See:
http://wyk.edu.hk/~paulsiu/Chemistry/ester.htm for the genreal reaction.
While the TEA looks like 3 alcohols hooked onto a nitrogen, it's the nitrogen that really takes the primary role in it's reactions. It's better to think of it as an ammonia that has had some of the hydrogens (in the case of TEA, all of them) replaced with something else. So TEA behaves very similar to ammonia.
That's why in a previous post I wrote this out for you:
"The ascorbic acid can donate 1 H+ to react with the negative charge on the amine in the triethanolamine (ROH)3N- (that's supposed to be a negative charge on the shorthand formula for a tri-alcohol amine). So the quick and easy answer is they are equivalents in an acid/base reaction and therefore you use equal normal weights. And since the equivalence is 1 - then your equivalent wieghts are equal to your molecular weights."
Anyway - I guess that wasn't enough.. Here's some more detail then -
The nitrogen in TEA has an electronegative charge, so it will grab onto H+ in acid solutions. It can then make acid salts, such as triethanolamine hydrochloride with solutions of hydrochloric acid. It's not a covalently bonded compound, but ionically bonded. In solution, expect this to behave like ionic compounds - the positive and negative sides of the compound will dissolve into the water than then float around independantly of each other...
With that info in mind, as the ascorbic acid dissolves into solution, it will liberate hydrogen ions. As you titrate with the TEA, the TEA will grab any of the H+ that was floating around free in the solution, and that the ascorbate ion would also just then be floating around as well. Only when they were crystallized or precipitated out of solution would they actually form a "compound", and I would expect that compound to be called triethanolamine ascorbate.
Infact, that is why TEA makes a basic solution when it is mixed with water. Think of water as a hydrogen ion (H+) and a hydroxide ion (OH-) that are ionically bonded to each other. When the TEA gets into solution, it will pull the H+ "away" from an OH-, and then you have the TEA ionically bonded to the H+ now, and some OH- floating around in solution because of that. It's the OH- that is now floating around that makes a solution of TEA dissolved in water basic.
It's just a simple acid-base reaction.