Review of Literature for Sensitizing Dyes
Ok, this is a work in progress, but this is the result of some armchair research I've done through, primarily, Google Books. It's a smattering of information relating to sensitizing dyes, and particularly those pre-1930ish.
Please download the word file for links, some quotations and a better sense of the context.
I don't claim to be making any conclusions here, but am rather just trying to get a base of information together. I recommend that people go through the links themselves and lets start discussions on certain topics. On the other hand, I don't know if we can conclude too much without some actual testing, but all this literature will certainly point us in the right direction and give us things to consider. In particular, some of the large reference lists should be very valuable for anyone wanting to go deep.
A lot of the literature is relating to bath sensitizing, much of which is done for astrophotography. There appear to be several contradicting statements in the various sources, so be careful in jumping to conclusions. Lastly, I've undoubtedly missed sources and indeed, haven't claimed to do an exhaustive search. I would simply search "pinacyanol", "orthochrome", or other dye names in Google books and follow the links which looked promising.
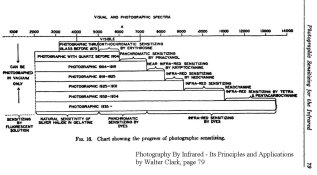
Here is a fairly complete list of all the sensitizers that are mentioned, posted mainly for the purpose of keyword searches. It'd be best to read the literature for broader context and use of the dyes . . . . .
Earliest Survey after Vogel:
- Ethyl Violet (triphenylmethane dye) - "red sensitizer for collodion"
- Eosin, Erythrosin, Rose Bengal (pyronine dyes) - "green and yellow sensitizers"
- Fast Red, Congo Red, Glycine Red, BenzoNitrol Brown (azo dyes)
- Acridine Orange, Alizarin Blue
Cyanine, a.k.a. quinoline blue
- 1,1'-Di-n-amyl-4,4'-cyanine iodide
In 1903, Miethe & Traube patented Ethyl Red (an isocyanine).
- 1,1'-Diethyl-2,4'-cyanine iodide
- sensitizer for green, yellow and orange
- a.k.a. chinaldin-ethyl-cyanin
In 1905, Homolka at Hoechst Dye Works discovered Pinacyanol.
- 1,1'-Diethyl-2,2'-carbocyanine iodide (or bromide, or chloride)
- Structure wasn't elucidated until mid-20's through research by Pope.
Dicyanine and Dicyanine A were manufactured later by Hoechst.
- 1,1'-Diethyl-2,4'-carbocyanine iodide (dicyanine)
- 6,6'-Diethoxy-1,1'-diethyl-2,4-carbocyanine iodide (dicyanine A)
Woolblack – a red sensitizer for gelatin
Pinaverdol - a green sensitizer
Orthochrom T
Pinachrom (pinachrom violet (?))
Homocol
Pinaflavol
Kryptocyanine (1919), Neocyanine (1925), Xenocyanine & other tricarbocyanines (1931) - IR sensitizers
In summation, “Pinacyanol, the most important sensitizer for the red, was used in all panchromatic materials until the thirties. Although by itself it gives fairly good sensitivity in the green in addition to its major contribution in the red, it was usually employed in conjunction with other dyes such as Orthochrome T, Pinaverdol, or pinachrome which conferred greater response in the green.”









 )
)