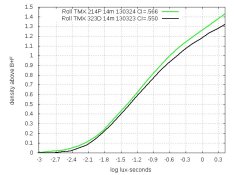
PE will be interested in this.
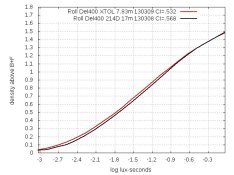
With 400-speed films, developer 214D produces a curve whose shape matches that of XTOL (example below), but is shifted down slightly. This shift doesn't matter when printing or scanning, but it makes the ISO-defined film-speed drop. Here are the curves for Delta 400. The left graph is the original curves; the right graph shows the 214D-curve shifted up by .02:
 -
- 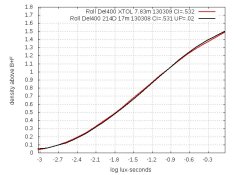
In the left graph, the 214D-curve crosses the Y=.1 line (.1 above B+F) about 1/2 stop to the right of the XTOL-curve, causing an apparent speed-loss of 1/2 stop. But the shifted curve in the right graph shows their shapes differ insignificantly. So how do I determine film-speed if a vertical curve-shift skews the ISO-number?
Long ago, the ISO standard used a superior method of determining film-speed called "Fractional Gradient" (FG). Stephen Benskin knows all about this. People complained that it was hard to calculate because you had to plot the curve, so ISO changed the standard to an approximation used to this day. I estimated the FG speeds for 214D, and always got at least 400 speed for 400-speed films. It's too bad that today's ISO method punishes a developer for shifting a curve down a little.
If you look at the right graph closely, you'll notice that 214D has a slight speed-loss wrt XTOL, but it's so small that I'll trade it for the finer grain I get. And the FG method says I'm still getting at least 400 speed, so I don't care. Or should I care? Any idea why this brew shifts the curve down a bit?
Some Test Results
Here are results of my testing so far. At a prior poster's request, I'm emphasizing low speed films because they gave Ryuji's DS-10 trouble. Pan-F+ failed with DS-10, but does fine with 214D (and D316).
[TABLE="width: 500"]
Film
Time
Tri-X
13.5
Tmax-100
13
Delta-100
12.25
Delta-400
18
Pan-F+
12.25
FP 4+
16
HP 5+
15
[/TABLE]
In my testing, I've seen two things worth noting:
1. The small downward curve-shift described above with 400-speed films.
2. An early shoulder (compensation) with Tmax-100. D316 did this too. Don't overexpose it. This compensation would be useful for compressing wide-scale scenes, but the cost you pay for this feature is lack of overexposure-latitude. This only applies to Tmax-100. All other films behave normally.
As always, comments are encouraged.
Mark Overton
With 400-speed films, developer 214D produces a curve whose shape matches that of XTOL (example below), but is shifted down slightly. This shift doesn't matter when printing or scanning, but it makes the ISO-defined film-speed drop. Here are the curves for Delta 400. The left graph is the original curves; the right graph shows the 214D-curve shifted up by .02:
 -
- 
In the left graph, the 214D-curve crosses the Y=.1 line (.1 above B+F) about 1/2 stop to the right of the XTOL-curve, causing an apparent speed-loss of 1/2 stop. But the shifted curve in the right graph shows their shapes differ insignificantly. So how do I determine film-speed if a vertical curve-shift skews the ISO-number?
Long ago, the ISO standard used a superior method of determining film-speed called "Fractional Gradient" (FG). Stephen Benskin knows all about this. People complained that it was hard to calculate because you had to plot the curve, so ISO changed the standard to an approximation used to this day. I estimated the FG speeds for 214D, and always got at least 400 speed for 400-speed films. It's too bad that today's ISO method punishes a developer for shifting a curve down a little.
If you look at the right graph closely, you'll notice that 214D has a slight speed-loss wrt XTOL, but it's so small that I'll trade it for the finer grain I get. And the FG method says I'm still getting at least 400 speed, so I don't care. Or should I care? Any idea why this brew shifts the curve down a bit?
Some Test Results
Here are results of my testing so far. At a prior poster's request, I'm emphasizing low speed films because they gave Ryuji's DS-10 trouble. Pan-F+ failed with DS-10, but does fine with 214D (and D316).
[TABLE="width: 500"]
[/TABLE]
In my testing, I've seen two things worth noting:
1. The small downward curve-shift described above with 400-speed films.
2. An early shoulder (compensation) with Tmax-100. D316 did this too. Don't overexpose it. This compensation would be useful for compressing wide-scale scenes, but the cost you pay for this feature is lack of overexposure-latitude. This only applies to Tmax-100. All other films behave normally.
As always, comments are encouraged.
Mark Overton