I've been pondering recent results:
* Concentrate 119P (aka Trial 20130119) (uses Phenidone) gives a hint worse grain with Tri-X than XTOL. With Delta-400, 119P is a hint better than XTOL.
* Concentrate D316 (also uses Phenidone) gives the same or a hint better grain with Tri-X than XTOL. This tells me that the higher level of ascorbate (or lower pH) improves grain with Phenidone.
* Concentrate 119D (same as 119P, but uses Dimezone S) gives slightly better grain with Tri-X than XTOL. That tells me that in this dev, Dimezone S is superior to Phenidone.
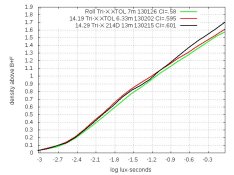
* Concentrate 214D (uses Dimezone S) gives significantly better grain with Delta-400 than 119P, and somewhat better than 119D. This tells me that the higher level of ascorbate (or lower pH) also improves grain with Dimezone S, and Dimezone S is already better than Phenidone. Here's a comparison of 214D and 119P with Delta-400:
119P:
214D:

214D is my best developer so far. Its formula is:
Propylene Glycol ..................... 12.2 g (grams, not ml)
Sodium Metaborate 4 mol ...... 1.8 g
Ascorbic Acid .......................... 4.2 g
Dimezone-S ............................ 0.08 g
A litre of developer contains 18 g of concentrate and 45 g of sodium sulfite with pH = 8.08.
The only difference between 214D and 212D that I posted a few days ago is that the Dimezone S in 214D was reduced to .08 g (instead of .09 g). This change makes the ascorbate/Dimezone ratio match XTOL. At this point, I plan try tweaking the amounts of metaborate and/or ascorbic acid to drop pH a little, and see what that does. Then I'll decide on a formula and run a bunch of tests on it. BTW, I found it wasn't necessary to resort to hydroquinone, because all the ascorbic acid still dissolves in this concentrate despite the lower level of metaborate. But I still want to try TEA instead of metaborate -- if the ascorbic acid will dissolve, it'll provide an interesting alternate to metaborate.
Actually, my tests show that the amount of Dimezone S makes little difference. 0.07 and 0.09 gave the same curve and grain as 0.08. I figured this meant the dev is regeneration-limited instead of dev-limited, so I reduced the ascorbic acid while keeping pH the same and surprise: It made no difference in dev-speed. I know sulfite also helps with regeneration, so perhaps it's the limiter? But I'm keeping sulfite at 45 g, which is half of what XTOL has, and doubling dev-time (by reducing pH) to get the same solvent-effect.
Good news: This insensitivity to Dimezone-quantity means that you can be sloppy about measuring Dimezone S. Sub-tenth-gram quantities are difficult to measure accurately without a milligram scale, so it's good to know that you can be 10% off and still be fine.
I was reminded that the supply of Dimezone S for us home-brewers might be unreliable. Alfa Aesar synthesized a batch, which Photographer's Formulary sells, but when that batch runs out, it's anyone's guess if they'll synthesize a new batch. Therefore, I suggest that you buy 100 g of Dimezone S from the Formulary. The price is reasonable, and that will give you a lifetime supply.
Mark Overton










 Actually, this is good news because it gives us a way of stressing a concentrate, and the same test can be done with the final developer. I suspect that the ratio of sodium metaborate to ascorbic acid largely determines vulnerability to crystallization, and the latest developer, 214D, has a lower ratio, so it's hopefully more robust.
Actually, this is good news because it gives us a way of stressing a concentrate, and the same test can be done with the final developer. I suspect that the ratio of sodium metaborate to ascorbic acid largely determines vulnerability to crystallization, and the latest developer, 214D, has a lower ratio, so it's hopefully more robust.
