Alan, this prompts me to realise that I don't really understand the processes whereby Sodium chloride solution can stabilise or even fix a salt print, or indeed any other Silver-based photographic material. I'm sure I must have read it somewhere but it's slipped away ...
The little I think I do know, I have gleaned from Mason and the Focal Encyclopaedia, and I have probably imperfectly understood them anyway.
Please comment and correct if necessary in what follows!
First, I think, in this context (chitchat about salted paper prints) at least, stabilisation and fixing are often used interchangeably, whereas for the photochemist, they are quite distinct processes.
(Further complicated by the fact that stabilisation has a quite different meaning in modern colour processing, where it is a process to protect the dyes and gelatin from deterioration; but as we're only talking about salted paper prints and other non-chromogenic processes I'll just leave that there).
Fixing is a process by which the undeveloped (or "un-printed-out"?) Silver halides are rendered into soluble compounds which dissolve out into the fixing solution, and the remnants of which are removed from the paper/gelatine/whatever in the subsequent washes.
Stabilisation is a process by which the Silver halides are rendered into insoluble but stable non-light-sensitive compounds, and no further washing takes place after stabilisation.
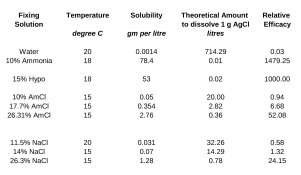
So, when we dunk an exposed salted paper print into a strong solution of Sodium chloride, what is actually happening to the remaining ("undeveloped" or "un-printed-out") Silver chloride? What complexes are formed, if any? Are they soluble, insoluble, stable, unstable, light-sensitive or what?
(Ware's article states that Silver chloride is left in the highlights of a salted paper print when stabilised with Sodium chloride solution, but he doesn't explain - there at least - what's happened to the rest of it)
As for experiments with "fixing" or "stabilising" modern materials with Sodium chloride solution, I wonder if the fact that they contain relatively little if any Silver chloride, with the bromide and iodide compounds being preferred, has any bearing? Or am I simply sniffing around at the bottom of a partially understood tree on my part?
(I love a smooshed-up metaphor.)