The pH of Vinegar is about 2.9 and is about a 1 molar solution (5% - 6%). Stops are in the range of 1% - 2% and are therefore much weaker than that and have lower buffer capacity. Many Stops are found to be in the pH range of 3.5 - 4.5 and many acid fixes are in the range of 4.5 - 5.5. Above that point, the fix is generally considered neutral in pH. A Stop must not be above 2% in concentration for good results.
The generation of Carbon Dioxide can take place in either a Stop or an acid Fix at pH values from 3.5 up to about 5.5. Above that, much of the CO2 will tend to stay in the water. The formation of pinholes from outgassing was never demonstrated to take place in tank or tray processing in any way with any film.
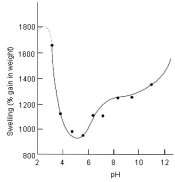
The matter of gelatin swell noted above is based on type of gelatin. Manufacturers use mainly bone Gelatin, not pig Gelatin. So the argument of swell vs pH is not one of hardness per se, but rather one of the type of gelatin used. Only the manufacturers can tell us for sure what type of gelatin is used. Kodak uses Bone Gelatin, Ilford and Fuji appear to use Bone Gelatin. The majority of photograde gelatins made today are Bone Gelatin. QG, I refer you back to a previous post here on swell vs pH.
I have fully discussed this item elsewhere and have discussed it with Bill both in person, on the phone, and on-line. Ian, you have commented here on 3rd party books many times.

My opinion of A&T is very high. I merely point this one bit out as an exception that would have been fixed in a new edition.
In no case has the neutralization been shown to cause problems with clumping nor is there any excessive heat in the emulsion. In fact, one of the engineers broke out in a fit of laughter when I brought it up. It is classed as "one of those old myths"!
PE










 G'Day!
G'Day!


