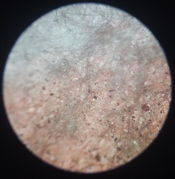
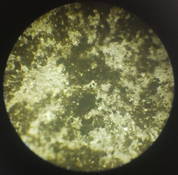
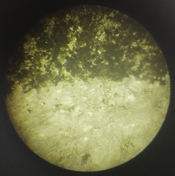
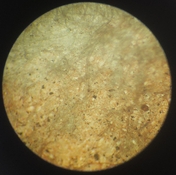
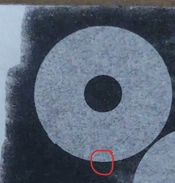
And I still find it very puzzling as to why I am getting positives when I use black pigment CMC+PVA (50:50), sulfamic acid (a pinch to ph3-4) and sodium benzoate. Here is an example of such a thing, I exposed this to 30 minutes under a 50W LED at 390nm. I sprayed 0.3% peroxide on it, then bathed it in dilute citric acid ph 4-5. Finally I sprayed the bejisus out of it for like 5 minutes in 50deg C water, scalding hot. How does that even work???
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
SPP Saidane's Print Process
-
AH
- Thread starter imgprojts
- Start date
Recent Classifieds
-
For Sale Canon RF 600mm f11, RF 100-400 Lenses
- Started by Jon Shiu
-
For Sale Fujifilm MAXIMA Glossy RA-4 Paper; Packs of 16x20 Sheets
- Started by Aidan Sciortino
-
Sold 1924 Carl Zeiss Jena 150mm f4.5 Lens in dial Compur
- Started by Jon Shiu
-
Found Plastic 5x7 film holders (Fidelity, Lisco)
- Started by blee1996
-
Want to Buy WTB: Digital timer for an enlarger
- Started by Terrence Brennan
Forum statistics
I've simplified the formula even more:
Mix 5% PVA (5g in100ml)
Emulsion:
2g PVA
0.2g FAO
0.1g Sodium Benzoate
Pigment
I've tested this with my washer shadowgraph testing system with good results with poster acrylic paint and with mica powder.
Except for black, you'll see a decidual image. You can easily coat over the previous exposure without damaging it. Just let it dry for long enough. Avoid painting it too thick or you'll get a really bad result. Thin coatings is the way to go.
After quite a bit of reading, as it turns out, I'm not the first person digging into this particular combo. Well maybe specifically for photography. But I've seen it mentioned in holographic forums replacing the PVA with gelatine and the FAO with FAC. But people mention PVA (PVOH) and FAO quite a bit. I've read a few papers regarding hydrogels made from PVOH crosslinked with Sodium Benzoate and in other ways and mixes including CMC and other polysachrides. There's a good long list of things that are both safe and cross linkable.
Mix 5% PVA (5g in100ml)
Emulsion:
2g PVA
0.2g FAO
0.1g Sodium Benzoate
Pigment
I've tested this with my washer shadowgraph testing system with good results with poster acrylic paint and with mica powder.
Except for black, you'll see a decidual image. You can easily coat over the previous exposure without damaging it. Just let it dry for long enough. Avoid painting it too thick or you'll get a really bad result. Thin coatings is the way to go.
After quite a bit of reading, as it turns out, I'm not the first person digging into this particular combo. Well maybe specifically for photography. But I've seen it mentioned in holographic forums replacing the PVA with gelatine and the FAO with FAC. But people mention PVA (PVOH) and FAO quite a bit. I've read a few papers regarding hydrogels made from PVOH crosslinked with Sodium Benzoate and in other ways and mixes including CMC and other polysachrides. There's a good long list of things that are both safe and cross linkable.
I've just discovered one more quirk. I tested a dilute FeCl + SB mix. This resulted in a faint image, hardly remarkable. Then I added FAO and the image started getting slightly more defined but no improvement with more FAO. each time things were slightly different until I realized that when I spray the 0.3% peroxide on the image it immediately starts to dissolve the image. So I then developed one by dunking it half way in the dilute acid bath only to watch it dissolve immediately. The trick is that as soon as the image is wet by the peroxide you immediately dunk it in acid. The faster you do it, the more color you keep. This is really a very interesting and maybe useful note. Maybe I totally screwed in making my peroxide. I got a 12% jug from HD and been using that diluted to make 0.3% 100ml at a time.
Having a conversation with Koraks I have a new thing to try this weekend. PVA + riboflavin + amine + acrylate. I got the riboflavin and amine coming tomorrow morning. Some have had success adding just those two ingredients but our friend Chad lasname Geepetee keeps insisting in adding an acrylate. As it turns out its not too expensive like $26 bucks for 16oz of MMA. According to this same friend, It can be used even at 5% total polymer volume. I then realized that water soluble 3D printing ink is basically acrylate monopolymer so I will try that. I'm not going to eat it, I mean I will try it in the photo-polymer. I got my new (new to me) 80's microscope setup with a 3W LED. I had to gut that thing out it had a huge heavy transformer inside. I replaced it with a USB wire, a latching switch and a 3W LED on an aluminum heatsink. total 10 to 30 grams vs a good half kilo of metal. I can get 400X without oil immersion and its cool! I have 100X for wide field and 1000X whenever I ever want to try oil immersion. There has to be something already known that can replace the bichromate stuff. My simple PVA formula seems to work OK for UV systems but not strong enough for use in a projector.
Here are some images of the multilayered PVA sample and another that was single layer. These are 100x. Also I started collecting all my used samples from last week I'm a single bottle lol. One test batch had new cyanotype formula apparently. It makes for an interesting possibility.. One go cyanotype+PVA+CMC. The CMC provides that weird positive part where the PVA doesn't dissolve.
Attachments
Oh...i think this is the reason I was getting positives:
It says there that you can make a polymerized gel such as PVA/CMC that contains metal ions such as Fe+3 mixed with a dye...such as the random dyes used in mica pigment depolymerize. Basically after exposure you can then wash off the illuminated areas and the non illuminated areas will stick around.
The PVA and CMC mix when mixed with FAO and citric acid then polymerizes maybe? And then the dye depolymerizes it when exposed to light. Its 5am, I've been reading patents trying to sleep, I could be just stretching my guessimetry.
It says there that you can make a polymerized gel such as PVA/CMC that contains metal ions such as Fe+3 mixed with a dye...such as the random dyes used in mica pigment depolymerize. Basically after exposure you can then wash off the illuminated areas and the non illuminated areas will stick around.
The PVA and CMC mix when mixed with FAO and citric acid then polymerizes maybe? And then the dye depolymerizes it when exposed to light. Its 5am, I've been reading patents trying to sleep, I could be just stretching my guessimetry.
I think we got something!!!
Actual values tested
• PVA – 3 spoons (5% solution in tap water PH controlled to 4 using sulfamic acid)
• FAO – 0.26 g
• Citric acid – 0.24 g
• Riboflavin – .03 g turns liquid orangy yellow but clear. hard to mix
• TEOA – 0.46g
• Acrylate (3D printing ink Sunlu water washable clear blue) – 1.03 g hard to mix starts as an unmixable luggy and turns mix into a foam after about a minute of mixing.
• Pigment – 0.19g to taste (well dispersed) Color Black mica ~35micron sheets
I think I can probably paint one sheet 8.5"X11" with this formula
Mixes well with only a slight 3D printing ink odor...but there an odor so it means there are fumes.
Result: Exposure to 380nm 50W at 3.5" distance for 3 minutes. Development 5 seconds peroxide spray 0.3% followed by very dilute (and black, very dirty full of previous inks and all sorts of oxalate residues) citric acid water. Evidently the exposure is way too much because the pigment clearly has light leaks from the outer rims of the washers I used. additionally comparing the before to the after, there was very little if any erosion of the dry mix matrix (I will compare under the microscope). It dries slightly tacky before exposure. very slightly tacky. The image can be seen as a sheen over the areas unexposed. The exposed areas become more matte. I was expecting the thing to work as a positive but I didn't follow my plan and just painted it and exposed it as I had done before with just the PVA. images to follow.
Actual values tested
• PVA – 3 spoons (5% solution in tap water PH controlled to 4 using sulfamic acid)
• FAO – 0.26 g
• Citric acid – 0.24 g
• Riboflavin – .03 g turns liquid orangy yellow but clear. hard to mix
• TEOA – 0.46g
• Acrylate (3D printing ink Sunlu water washable clear blue) – 1.03 g hard to mix starts as an unmixable luggy and turns mix into a foam after about a minute of mixing.
• Pigment – 0.19g to taste (well dispersed) Color Black mica ~35micron sheets
I think I can probably paint one sheet 8.5"X11" with this formula
Mixes well with only a slight 3D printing ink odor...but there an odor so it means there are fumes.
Result: Exposure to 380nm 50W at 3.5" distance for 3 minutes. Development 5 seconds peroxide spray 0.3% followed by very dilute (and black, very dirty full of previous inks and all sorts of oxalate residues) citric acid water. Evidently the exposure is way too much because the pigment clearly has light leaks from the outer rims of the washers I used. additionally comparing the before to the after, there was very little if any erosion of the dry mix matrix (I will compare under the microscope). It dries slightly tacky before exposure. very slightly tacky. The image can be seen as a sheen over the areas unexposed. The exposed areas become more matte. I was expecting the thing to work as a positive but I didn't follow my plan and just painted it and exposed it as I had done before with just the PVA. images to follow.
One more comment. I literally jetted my focet on to the image and it resisted. Below you can see the mixture after adding all the active ingredients. You can see the before and after. I'm going to try a shorter exposure to see if that increases sharpness or of it just washes away.
Attachments
Yup it sharpened a bit but I still see some light bleed. No apparent degradation of the ink on the exposed side. Normally with the FAO only I develop quick or else the image starts to run. But here the image was shot with warm water jet. Not a little trickle, we're talking water splashing out of the sink. I though maybe the bleed part would go away if I used a more aggressive jet.
Attachments
OK, I got significant developments literally. Last night I prepared some samples of the new polymer formula. I added 1ml of water and made 3 samples, then added fine carbon in addition to the mica powder and made 3 samples, then I added 1g CMC and made 3 samples and finally added .1g of sodium Benzoate and made 3 samples.
I took one sample of each and left it in the bathroom under very bright LED light. Phillips ultra definition daylight, so slightly blue in my opinion, but doesn't matter. After 8 hrs this morning I exposed all four of those for 1m of 380nm LED light. The result were very nicely defined lines.
I took one sample of each and left it in the bathroom under very bright LED light. Phillips ultra definition daylight, so slightly blue in my opinion, but doesn't matter. After 8 hrs this morning I exposed all four of those for 1m of 380nm LED light. The result were very nicely defined lines.
Attachments
So I through interesting.. I noticed a much more yellowish tinge in all samples and I exposed them all wet for 5 minutes same UV lamp. They all turned orange. But then I took one sample and dunked it in dilute HCL. The orange immediately dissolved.
Attachments
Next, I looked at exposure times. Having already gone from 3 minutes to 1 minute, I tested 30 seconds, 10 seconds, 5seconds and here are the results from left to right. The result was the same with little to no loss of material under a strong water jet.
Attachments
First image! I exposed on my projector for 5 minutes. Normally for new cyanotype the exposure is 15 minutes. There's definetly an image in there. Its supposed to be a trolley on a busy urban street.

The speed of this emulsion is about 5 seconds. its too fast. the previous image was over exposed and to even see the trolley I ended up washing most of the central part of the image. The sides where there was no direct light don't clear to paper white. The other thing is that clearing take a long long time. I just keep getting more and more yellow water for tens of minutes. So I will need to figure out a way to slow the process down a bit. I've just finished dilution the active ingredients in 50ml of water and the using only 1ml. The result after 1m exposure was a perfect exposure as before, but somehow hard to clear. I used poster paint this time. That may be the reason. But wow, 1/50th. Except for the Sunlu stuff which I kept the same ratio as before. Maybe I need to reduce that too.
Exposing the new samples for 5 minutes with 1/5th the activator does not yellow tinge the paper as it did before!
New mix:
Activator:
50ml water
• Riboflavin – .01 g turns liquid orange yellow but clear. hard to mix
• FAO – 0.14 g
Ph measures ~5 keep Ph at around 4
• TEOA – 0.49g - liquid becomes dark orange and has Ph ~9
Citric acid – 0.65 g -liquid is light yellow and has a Ph ~4
Emulsion:
1.93g PVA
.34g Sunlu makes a very milky white mix
.96 poster paint
1ml of activator
I think for anyone using a UV lamp this stuff works exactly (very differently) than Gum Bichromate.
1) First off, no yellowing
2) relatively safe-er except for the Sunlu stuff
3) you can make the coatings really thick in ink and they got staying power. I literally tried to brush the material off on this reduced activator and I got nothing to come off. This could be good and bad. I assume that at some point the polymerization would be weaker. I think I will play with the Sunlu stuff and try to dilute it in alcohol and use a smaller portion with more control that way. Currently Anycubic for example is selling their 1000g bottle for 15 bucks. For a Joe Shmoe like me that's an infinite amount of prints if all I need is .5g for a few prints. Infinity is 2000 prints . But I think this material is swappable with more inexpensive materials.
. But I think this material is swappable with more inexpensive materials.
Anyway, just like that I am proved wrong, no, infinity is not 2000 prints but 40,000 prints because I just diluted the entire emulsion from 1ml to 20ml and guess what, it still worked at 1minute exposure minus the poster paint stain on the paper. Anyway, now I guess the challenge is to somehow come up with a decent image...ie, the process. Clearly the chemistry works, but what is the proper way to apply, expose and develop it and how to do that for multi-layer image.
New mix:
Activator:
50ml water
• Riboflavin – .01 g turns liquid orange yellow but clear. hard to mix
• FAO – 0.14 g
Ph measures ~5 keep Ph at around 4
• TEOA – 0.49g - liquid becomes dark orange and has Ph ~9
Citric acid – 0.65 g -liquid is light yellow and has a Ph ~4
Emulsion:
1.93g PVA
.34g Sunlu makes a very milky white mix
.96 poster paint
1ml of activator
I think for anyone using a UV lamp this stuff works exactly (very differently) than Gum Bichromate.
1) First off, no yellowing
2) relatively safe-er except for the Sunlu stuff
3) you can make the coatings really thick in ink and they got staying power. I literally tried to brush the material off on this reduced activator and I got nothing to come off. This could be good and bad. I assume that at some point the polymerization would be weaker. I think I will play with the Sunlu stuff and try to dilute it in alcohol and use a smaller portion with more control that way. Currently Anycubic for example is selling their 1000g bottle for 15 bucks. For a Joe Shmoe like me that's an infinite amount of prints if all I need is .5g for a few prints. Infinity is 2000 prints
 . But I think this material is swappable with more inexpensive materials.
. But I think this material is swappable with more inexpensive materials.Anyway, just like that I am proved wrong, no, infinity is not 2000 prints but 40,000 prints because I just diluted the entire emulsion from 1ml to 20ml and guess what, it still worked at 1minute exposure minus the poster paint stain on the paper. Anyway, now I guess the challenge is to somehow come up with a decent image...ie, the process. Clearly the chemistry works, but what is the proper way to apply, expose and develop it and how to do that for multi-layer image.
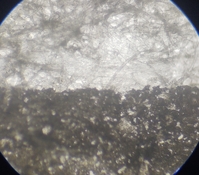
Here's a microscope view one of the dilute samples. This one has carbon. Looking at the stain vs the covered area one can clearly see that the non paper white is due to some sort of stain that just wicks into the paper fibers. If I coat the paper first with a hardened PVA coat maybe that will clear. The tests that use mica only do clear because the color is in large particles that get stuck above the paper fibers.
Attachments
Without giving this much thought, it occurred to me that CMC sucks at flowing anywhere. So after dilution to 20ml, I've added 5 grams of CMC and 4 grams of fine carbon pigment. And I tried this with the original one gram of pigment too. You get paper white with a heavy pigment load and hopefully a less sensitive image forming formula??? On the right you see the first formula that was uber loaded with pigment , resin and activator. On the left you see the latest formula.
Attachments
Looking at breaking the formula, here's my first experiment. Mixed PVA with black mica. Then I started adding ingredients sequentially. Not surprisingly nothing happens until there's monomer. This was a 1 minute exposure per sample the sample where I added the TEOA simply washed right off unexpectedly. Surprisingly the monomer was not the strongest. When changing the pH to just some level of acidity that's when it polymerized the best. I'm testing the addition of FAO and then I will change the order of mixing to try the strongest items first.
Attachments
In today's formula adjusting experiment, I tested PVA + Pigment + (1/3)*3D resin. By the time I developed the whole thing was polymerized so no image was formed. To the same formula then I added 20ml of water and 1ml of activator. This painted very faintly and had a faintly but visible result. I added 5 grams of CMC and 4 grams of carbon pigment and that gave me a good image just like before. Because I used 0.09g of monomer, 1/3 the amount I did before, this seems to have affected the strength and or speed of the emulsion because this time I was able to wash some of it with the water jet stream. Per one of the papers I've been reading, the reaction rate R_p is proportional (not equal):
R_p = k · [M]^2 ·^0.5 · [R]^0.5 · [O2]^0.5
where
[M] = Monomer concentration (Sunlu 3D printing monomer)
= Sensitizer concentration (Riboflavin)
[R] = Reducing agent concentration (TEOA, FAO)
[O2] = Oxygen concentration (assumed constant here)
k = Proportionality constant
So reducing the monomer to 1/3 had a big impact of around 56% lower R_p, just like when I diluted the sensitizer. The sensitizer has a sub-linear proportionality, while the monomer amount has a quadratic proportion, so diluting from 1ml to 50ml had an 86% decrease in reaction rate if the formula holds. I was trying to go for 0.01g monomer but my scale does not have the resolution to go that low. instead, it appears I will have to use a measurable amount and then dilute it in PVA and water for use like the activator. The activator so far has survived a few days in an orange medicine bottle.
R_p = k · [M]^2 ·
where
[M] = Monomer concentration (Sunlu 3D printing monomer)
[R] = Reducing agent concentration (TEOA, FAO)
[O2] = Oxygen concentration (assumed constant here)
k = Proportionality constant
So reducing the monomer to 1/3 had a big impact of around 56% lower R_p, just like when I diluted the sensitizer. The sensitizer has a sub-linear proportionality, while the monomer amount has a quadratic proportion, so diluting from 1ml to 50ml had an 86% decrease in reaction rate if the formula holds. I was trying to go for 0.01g monomer but my scale does not have the resolution to go that low. instead, it appears I will have to use a measurable amount and then dilute it in PVA and water for use like the activator. The activator so far has survived a few days in an orange medicine bottle.
ohh... bingo! with my 380nm I get perfectly fine circles but my 3000lm with very intense light takes like 15 minutes to produce a very faint image. A very strong flashlight with a blue filter produced faded circles within 5 minutes of exposure. So it looks like actually I do need the speed and also I need a sensitizer that reacts more to visible light. So I think that between riboflavin and Ferric Ammonium Oxalate there is level that has to be balanced. That or I need to work on swapping the projector light to an LED. this is actually really exciting, it means the emulsion was working all along and I just need the right wavelength!
This morning I'm just thinking about the way this works. According to my observations on the microscope, this general "gum bichromate" process is practically a one layer process. The colored particles don't actually penetrate into the paper. When the particles are too small and they do go into the paper, then you got a stain that you cannot remove due to physics. For any given flow the velocity at the wall is zero, so no matter what you do, you cannot push the particles off the paper fibers. Using the circle samplers I can tell that very fine particles ~1um are best for higher contrast. Smaller particles go into the water color paper fibers and also block UV light. The particles have to be suspended in something above the paper. That means that UV light must pass thru between the particles to fuse the glue/hydro-gel/polymer onto the paper while at the same time trapping the particles. CMC's role in some of my experiments has been to form a very viscous gel that you can load with smaller than 1um particles without allowing the particles to flow into the paper. Even with large mica particles up to 50um in size, the gel is penetrated by UV and the particles are suspended, but Also there are passages between each particle allowing the light to pass thru below. So there is a saturation point where if you add more particles, the glue only cures on the surface layer and it peals off. "Shades" of color in this process merely come from more or less particles. So that means that the process has to be slow enough to allow some areas to not end up as strong as other areas. If you make an emulsion that is too strong, you'll get very high contrast but only areas fully covered will be paper white. Chemical polymerization can also be kicked off. So for visible light, you want this particular FE+3/monomer coating to be very close to the point where polymerization is triggered but not so close that the polymerization is triggered chemically. Accordingly, the coating cannot be bone dry or nothing will happen??? but it cannot be wet as in my experiment with gelled gelatin because then local polymerization will travel sideways chemically. Anyway, probably nobody cares, but these are my notes I keep on an ever growing Joplin notebook. On an unrelated note, I realize how illprepared I have been writing my notes. I did some diggings and found this thing: https://www.elabftw.net/ its a note taking tool for lab stuff. I wonder if anyone else is using it for this sort of thing.
Now I'm trying to shift the sensitivity closer to visible range and it seems to be working. Below are two images. One is NewCyanotype suspended in CMC which gives it glorious detail. The other is my latest formula. I need to cut down on riboflavin and CMC. But look at that! It happened so suddenly... Here I am panning another meaningless piece of paper that seems to have over exposed, them BAM! I see a trolley!... Cars, reflection on the road, the clock on the right. Jubilation!
Attachments
The cyanotype is the blue one...lol
I'm continuing to look for ways to make the reaction more visible light reactive. Looks like L-arginine, which acts like the little blue pill if you eat it, can intensify the photo reactivity of riboflavin. Its cheap since you only need a tiny 3 to 5% of total solids. So at some point I'll be testing PVA+CMC+pigment as the base matrix plus 3D printing monomer plus the sensitizer, FAO+Riboflavin+L-arginine+TEOA+sodium benzoate+citric acid.
Thru my poor understanding, PVA, CMC, Gum Arabic, Gelatin are all just along for the ride but provide a temporary way to suspend the pigment in place and prevent it from staining the paper. FAO is there because when it encounters a free radical or becomes a free radical it can crosslink the matrix a little. Riboflavin kicks off free radicals with visible light and help from L-arginine and TEOA. The free radicals polymerize the 3D printing Acrylate. Meanwhile Sodium Benzoate helps to keep the radicals alive for longer. Citric acid chalates the ferric ions and regenerates riboflavin so it can do it's thing again.
I probably have it all wrong so it would be cool if someone else would review. This mixes ferric ion crosslinking with dye polymerization. All ingredients seem to be relatively safe to use except for the acrylate. The stuff works well with UV but to be able to use visible light projectors we need to push the reactivity closer. I don't want to end up with a dark room either so keeping reactivity close to blue is good enough.
Thru my poor understanding, PVA, CMC, Gum Arabic, Gelatin are all just along for the ride but provide a temporary way to suspend the pigment in place and prevent it from staining the paper. FAO is there because when it encounters a free radical or becomes a free radical it can crosslink the matrix a little. Riboflavin kicks off free radicals with visible light and help from L-arginine and TEOA. The free radicals polymerize the 3D printing Acrylate. Meanwhile Sodium Benzoate helps to keep the radicals alive for longer. Citric acid chalates the ferric ions and regenerates riboflavin so it can do it's thing again.
I probably have it all wrong so it would be cool if someone else would review. This mixes ferric ion crosslinking with dye polymerization. All ingredients seem to be relatively safe to use except for the acrylate. The stuff works well with UV but to be able to use visible light projectors we need to push the reactivity closer. I don't want to end up with a dark room either so keeping reactivity close to blue is good enough.
FAO is there because when it encounters a free radical or becomes a free radical it can crosslink the matrix a little. Riboflavin kicks off free radicals with visible light and help from L-arginine and TEOA. The free radicals polymerize the 3D printing Acrylate. Meanwhile Sodium Benzoate helps to keep the radicals alive for longer.
Thanks for the update.
I gathered that on exposure to 300 - 450nm (?) radiation many ferric (Fe3) components (among which FAO, FAC etc.) are being reduced to "ferrous" (Fe2). The ferrous is reacting with hydrogen peroxide by generating free radicals.
So in order to polymerize a monomer, you do not necessarily need Riboflavin. Well, it depends on your light source. By the way, have you ever considered using a laser source? 405nm and particularly, 450nm laser diodes have become very affordable.
In my experience and speaking for non-UV light sources, FAO is performing very well at 405nm. At 450nm though switching to FAC might be a better option.
I probably have it all wrong so it would be cool if someone else would review. This mixes ferric ion crosslinking with dye polymerization. All ingredients seem to be relatively safe to use except for the acrylate. The stuff works well with UV but to be able to use visible light projectors we need to push the reactivity closer. I don't want to end up with a dark room either so keeping reactivity close to blue is good enough.
Regarding that "acrylate" stuff, I assume it is "water washable" but NOT water soluble. Is that correct?
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |