Thank you very much for your comments. I really appreciate it.
I understand. If residual halides remain, they can darken when exposed to ultraviolet light. Therefore, even if the plate is varnished after being likely rehalogenated, it might be inevitable for it to eventually darken due to UV reactions.
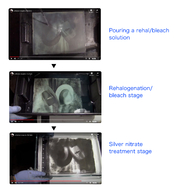
You might already know, but in the wet plate collodion process, Copper Intensification involves rehalogenating a plate that has been fixed and washed, using a Copper bleach solution (potassium bromide + Copper sulfate), and then immersing it in a Silver nitrate solution for redevelopment. After redevelopment, the plate is not exposed to sunlight. Copper bleach and silver nitrate redevelopment are conducted in a well-lit room.
In the first place, I've been researching why such chemical reactions occur, but I don't understand despite searching online. Do you know about this mechanism?
In any case, after redevelopment, the plate is rinsed and dried without being fixed again. This seems to be a common method that many wet plate photographers use to achieve the necessary density for making POP prints like Salted paper.
Regarding my question, I was inquiring whether the image would darken if only copper bleach is used without silver nitrate redevelopment. Again, as you suggested, due to the presence of residual halide silver after copper bleach, it seems that the image will indeed darken over time due to the effects of ultraviolet light. I am planning to conduct some experiments with the plates that I recently made.
Also, in a few previous posts, Nicholas Lindan suggested using B&W stabilizer. With this method, would it be possible to fix the rehalogenated silver and avoid darkening due to ultraviolet light? I'm interested in trying out this approach as well.
Thanks again.
I understand. If residual halides remain, they can darken when exposed to ultraviolet light. Therefore, even if the plate is varnished after being likely rehalogenated, it might be inevitable for it to eventually darken due to UV reactions.
You might already know, but in the wet plate collodion process, Copper Intensification involves rehalogenating a plate that has been fixed and washed, using a Copper bleach solution (potassium bromide + Copper sulfate), and then immersing it in a Silver nitrate solution for redevelopment. After redevelopment, the plate is not exposed to sunlight. Copper bleach and silver nitrate redevelopment are conducted in a well-lit room.
In the first place, I've been researching why such chemical reactions occur, but I don't understand despite searching online. Do you know about this mechanism?
In any case, after redevelopment, the plate is rinsed and dried without being fixed again. This seems to be a common method that many wet plate photographers use to achieve the necessary density for making POP prints like Salted paper.
Regarding my question, I was inquiring whether the image would darken if only copper bleach is used without silver nitrate redevelopment. Again, as you suggested, due to the presence of residual halide silver after copper bleach, it seems that the image will indeed darken over time due to the effects of ultraviolet light. I am planning to conduct some experiments with the plates that I recently made.
Also, in a few previous posts, Nicholas Lindan suggested using B&W stabilizer. With this method, would it be possible to fix the rehalogenated silver and avoid darkening due to ultraviolet light? I'm interested in trying out this approach as well.
Thanks again.