I really doubt that; I'd expect the opposite. Higher pH means in principle there's more opportunity for the developing agent to oxidize and be rendered inactive. In rodinal, a copious amount of sulfite is present to provent this from happening.
The hydroxide content will need to be set such that the target pH of the working strength developer is met; this by necessity will have to be the same pH for the foma and the adox product; both are supposed to Rodinal variants/clones, after all, so logically the working strength solution will need to be equivalent. Both are based on potassium hydroxide so we can quite safely assume that the amount of hydroxide in both these products will be roughly the same.
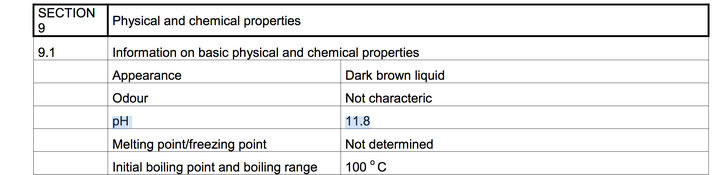
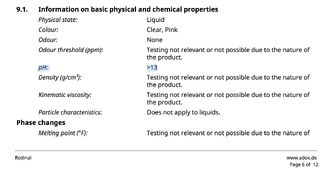
The difference in pH is likely due to the effect of adjuvants (note e.g. the zinc powder) in the bottled mix; I personally wouldn't pay much attention to the pH of a rodinal concentrate since it can be qualified in any case as "godawful high". In the end, it doesn't really matter whether it's nearly 12 or over 13; either way it's as alkaline as things can realistically get.