cirwin2010
Member
I've been having issues with palladium toner staining my paper once placed in the fixing bath. To rule out that this is not due to any of the steps in my process for Kallitypes, I performed the expirment below. I do want to stress that I ONLY put paper directly into the toner. No sensitizer, development, or clearing bath were used for my tests. The issue is exclusively with the toner, fixer, and paper.
1. Soak a piece of fresh paper in the palladium toner for 15 minutes
2. Rinse for 20 seconds
3. Cut paper in half
4. Fix half of paper for 2 minutes
5. Compare fixed paper with unfixed paper.
Palladium toner formula:
-1L water
-10g citric acid
-15 drops of Sodium Chloropalladite 15% solution
Fixer formula:
-1L water
-50g of sodium thiosulfate
Paper:
Legion Revere Platinum
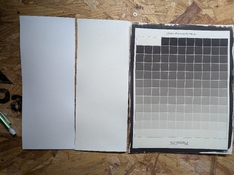
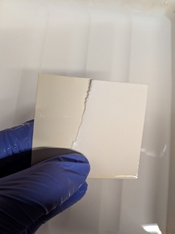
As you can see in the attached image (where I am holding the paper), there is a very distinct yellow stain. This only appear after the paper enters the fixer and gets worse when the paper dries and is unsightly (see image of test strips). The stain is present on the front and back of the paper and almost looks like tea toning. It does not wash out once stained despite by best efforts.
I did a bunch of testing and came to the following conclusions:
1. Stain is dependent on toner strength (fresh vs exhausted)
2. Stain is dependent on duration in toner (5 minutes yeilds less stain than 15 minutes)
3. Stain is dependent on wash prior to fixing (less stain with longer wash time -- I could not completely clear the paper)
4. Stain is NOT dependent on fix time (appears immediately)
5. Platinum toner (Potassium Tetrachloroplatinate) does NOT stain the paper
6. Stain is paper dependent (Hahnemühle Platinum Rag has comparatively minimal stain)
My best guess is the palladium salt is getting embedded (or reacting) with the paper fibers or paper sizing then reacting with the sodium thiosulfate to form either metallic palladium or some other visible, insoluble compound. But I'm not a chemist so I couldn't say for sure.
Has anyone managed to solve this problem? I did order some amount of palladium solution instead of platinum because it was cheaper... oops?
1. Soak a piece of fresh paper in the palladium toner for 15 minutes
2. Rinse for 20 seconds
3. Cut paper in half
4. Fix half of paper for 2 minutes
5. Compare fixed paper with unfixed paper.
Palladium toner formula:
-1L water
-10g citric acid
-15 drops of Sodium Chloropalladite 15% solution
Fixer formula:
-1L water
-50g of sodium thiosulfate
Paper:
Legion Revere Platinum
As you can see in the attached image (where I am holding the paper), there is a very distinct yellow stain. This only appear after the paper enters the fixer and gets worse when the paper dries and is unsightly (see image of test strips). The stain is present on the front and back of the paper and almost looks like tea toning. It does not wash out once stained despite by best efforts.
I did a bunch of testing and came to the following conclusions:
1. Stain is dependent on toner strength (fresh vs exhausted)
2. Stain is dependent on duration in toner (5 minutes yeilds less stain than 15 minutes)
3. Stain is dependent on wash prior to fixing (less stain with longer wash time -- I could not completely clear the paper)
4. Stain is NOT dependent on fix time (appears immediately)
5. Platinum toner (Potassium Tetrachloroplatinate) does NOT stain the paper
6. Stain is paper dependent (Hahnemühle Platinum Rag has comparatively minimal stain)
My best guess is the palladium salt is getting embedded (or reacting) with the paper fibers or paper sizing then reacting with the sodium thiosulfate to form either metallic palladium or some other visible, insoluble compound. But I'm not a chemist so I couldn't say for sure.
Has anyone managed to solve this problem? I did order some amount of palladium solution instead of platinum because it was cheaper... oops?
Attachments
Last edited: