Is the noise you observe random by nature and does the value change quickly? If so, the value could be averaged using analog (RC circuit) or digital (if the system is later connected to PC) system to obtain even more stable data.
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
- Home
- Forums
- Analog Workflow Forums (100% Analog/Traditional)
- Darkroom
- Silver Gelatin Based Emulsion Making & Coating
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Measuring vAg - preliminary results!
-
A
- Thread starter Photo Engineer
- Start date
Recent Classifieds
-
For Sale 11x14 Premier Contact Printing Frame
- Started by 2Dogs
-
For Sale Bronica SQ Lot - 3 Bodies, Lens, More! $850 Free Shipping in USA.
- Started by indy_kid
-
For Sale Jobo CPP2 with Lift, 3063, 2830, and 1530 tanks - $1200
- Started by aconbere
-
Want to Buy 4x5 vacuum film holder
- Started by fdelconte10
-
For Sale Mamiya TLR System Prism Finder - Cased
- Started by davela
Forum statistics
Well, actually, I can damp out the noise in 2 ways. One is by using an analog meter. Then the inertia of the needle damps out digital fluctuations (another benefit of analog). The needle simply does not move fast enough. Another way is to ballast the circuit by using 2 1.5 V AA batteries and a pot and center the reading on zero. In the mv range, and with such low current, the system is hypersensitive. Waving the probes in the air can give me readings varying from -100 to +100 mv sometimes, but it varies with the phase of the moon. 
At EK we used some sort of custom amplifier and lots of grounds to prevent this type of noise. At home, I am just improvising. Unfortunately, I never knew the contents of the black boxes we used.
Another part of the noise is the conditioning of the Silver electrode and the cleanliness. We "bought" our electrodes prepared for us, and then conditioned them with standard salts and used another black box. Again, I am improvising as I go along.
I should mention that there are 2 types of noise, if you read into the above what it implies. There is a rapid fluctuation back and forth and a slow drift up or down. The former is due to the low current and voltage, and the latter is due to conditioning and deconditioning of the electrode.
There is another separate problem, and that is with my Potassium Nitrate. It is not pure enough and tests positive for a tiny level of KCl in it. This affects the action of the bridged system during long use. It is used very dilute though, which reduces or minimizes the effect of the impurity on the system during measurement.
However, purchase of pure KNO3 is restricted in the US at the present time. So, I have to use what I can get right now.
PE

At EK we used some sort of custom amplifier and lots of grounds to prevent this type of noise. At home, I am just improvising. Unfortunately, I never knew the contents of the black boxes we used.
Another part of the noise is the conditioning of the Silver electrode and the cleanliness. We "bought" our electrodes prepared for us, and then conditioned them with standard salts and used another black box. Again, I am improvising as I go along.
I should mention that there are 2 types of noise, if you read into the above what it implies. There is a rapid fluctuation back and forth and a slow drift up or down. The former is due to the low current and voltage, and the latter is due to conditioning and deconditioning of the electrode.
There is another separate problem, and that is with my Potassium Nitrate. It is not pure enough and tests positive for a tiny level of KCl in it. This affects the action of the bridged system during long use. It is used very dilute though, which reduces or minimizes the effect of the impurity on the system during measurement.
However, purchase of pure KNO3 is restricted in the US at the present time. So, I have to use what I can get right now.
PE
Well, the noise is gone as are most of the other problems. It was the conditioning that was not sufficient.
Here are the correct conditions for 12 guage Silver wire. They are stronger for the 1 oz billet.
4 minutes treatment in 1 M NaBr with 3.5 V from two AA batteries. The Silver electrode you are going to use is connected to the positive (red) pole of the batteries and the negative pole (black) goes to the donor electrode which is also Silver of the same purity. The Silver electrode will blacken significantly during this 4 minute conditioning / plating. The electrode must be then washed with distilled water and must be stored under distilled water. It will last for at least 24 hours. After that, the readings begin to become unstable again.
From the start of the conditioning, the operation and storage must be under reduced light as you are creating a light sensitive surface on the Silver electrode.
Insure that all sides of the electrode are plated evenly and slowly over 4 minutes. If you plate more than about 12cm of #12 wire, you may have to use longer times or higher voltages. I find that a 20 gram billet requires 9V over about 3 - 4 minutes.
If your readings are low, you have overdone the conditioning, and if they are high, you have underdone the plating. You must have an even coating on the Silver electrode which does not flake off during use. This was one of my problems.
My results in two tries? 1: -115.5 and 2: -116.1, Calculated is -116.96. These were at 20 deg C using 1.0 M NaBr with stirring.
Cleanliness and proper storage along with the right conditioning seems to have fixed up all problems. My thanks to Kirk Keyes for 6 inches of 99.99 fine #12 Silver wire.
PE
Here are the correct conditions for 12 guage Silver wire. They are stronger for the 1 oz billet.
4 minutes treatment in 1 M NaBr with 3.5 V from two AA batteries. The Silver electrode you are going to use is connected to the positive (red) pole of the batteries and the negative pole (black) goes to the donor electrode which is also Silver of the same purity. The Silver electrode will blacken significantly during this 4 minute conditioning / plating. The electrode must be then washed with distilled water and must be stored under distilled water. It will last for at least 24 hours. After that, the readings begin to become unstable again.
From the start of the conditioning, the operation and storage must be under reduced light as you are creating a light sensitive surface on the Silver electrode.
Insure that all sides of the electrode are plated evenly and slowly over 4 minutes. If you plate more than about 12cm of #12 wire, you may have to use longer times or higher voltages. I find that a 20 gram billet requires 9V over about 3 - 4 minutes.
If your readings are low, you have overdone the conditioning, and if they are high, you have underdone the plating. You must have an even coating on the Silver electrode which does not flake off during use. This was one of my problems.
My results in two tries? 1: -115.5 and 2: -116.1, Calculated is -116.96. These were at 20 deg C using 1.0 M NaBr with stirring.
Cleanliness and proper storage along with the right conditioning seems to have fixed up all problems. My thanks to Kirk Keyes for 6 inches of 99.99 fine #12 Silver wire.
PE
Well, this is more good progress!
I think the conditioning and keeping the "sensitived" electrode in the dark is a great tip that one may not ever figure out on their own...
You say "reduced light" - do you mean safelights or are dim lights good enough?
Any reason not to use a stainless or carbon rod for the "donor" electrode/cathode (negative terminal of the battery)?
If anyone is interested where I found the 12 gauge silver wire, it was here:
http://www.ccsilver.com/silver/superfines.html#four - follow the link for the ".9999 wire"
I think the conditioning and keeping the "sensitived" electrode in the dark is a great tip that one may not ever figure out on their own...
You say "reduced light" - do you mean safelights or are dim lights good enough?
Any reason not to use a stainless or carbon rod for the "donor" electrode/cathode (negative terminal of the battery)?
If anyone is interested where I found the 12 gauge silver wire, it was here:
http://www.ccsilver.com/silver/superfines.html#four - follow the link for the ".9999 wire"
I used subdued light and it seemed to work just fine. This is a two bulb system in my lab as opposed to the 8 bulbs I normally use, so it is a lot less light.
I wanted to go down stepwise.
As for the other electrode, I am not sure. I used silver to keep contamination at a minimum. All I can say is "give it a try". I will be trying other materials myself. I would not recommend "poison" metals though such as copper, zinc, lead and tin, and I would worry about stainless, as Iron contamination will fog emulsions as well. Some carbon has adsorbed sulfur compounds that are not nice either. It depends on the source of the carbon. So, testing is obviously in order. Since plating is taking place, I try to avoid anything that might be carried over in the stream to plate out.
So, the bottom line is "use what works". I know Silver works.
PE
I wanted to go down stepwise.
As for the other electrode, I am not sure. I used silver to keep contamination at a minimum. All I can say is "give it a try". I will be trying other materials myself. I would not recommend "poison" metals though such as copper, zinc, lead and tin, and I would worry about stainless, as Iron contamination will fog emulsions as well. Some carbon has adsorbed sulfur compounds that are not nice either. It depends on the source of the carbon. So, testing is obviously in order. Since plating is taking place, I try to avoid anything that might be carried over in the stream to plate out.
So, the bottom line is "use what works". I know Silver works.
PE
Good thougts.
I had used carbon rods from dry cell batteries when I last played with electrolosis (about 30 years ago).
I can see why not to use poison metals, however, I would think stainless should be OK as any iron leached from it should be headed right back to the electrode from the current in the solution... Anyway, I guess I've got the silver wire around to use. I guess platinum wire would work well too, and I've always wanted to get a bit of that for various projects...
I had used carbon rods from dry cell batteries when I last played with electrolosis (about 30 years ago).
I can see why not to use poison metals, however, I would think stainless should be OK as any iron leached from it should be headed right back to the electrode from the current in the solution... Anyway, I guess I've got the silver wire around to use. I guess platinum wire would work well too, and I've always wanted to get a bit of that for various projects...
Some photos
I am now at the point of being able to replicate results to within about +/- 5 mv, but with a slope adjustment as the solution becomes more dilute. I am working on that. It may be due to having immersed too little of the billet in the electrolyte.
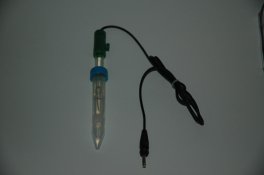
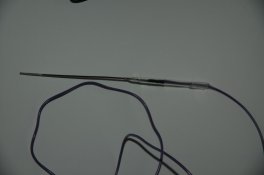
Here are photos of the reference electrode, the billet, the bridge and a trial setup of a beaker ready for precipitation of a real emulsion. The reference electrode is immersed in its conditioning/storage solution, and the bridge is also immersed in its own gel/salt storage solution. The bridge is marked as to polarity and the salt that it is to be used with. These are two critical items in this measurement.
The making beaker in the 4th picture contains 2 inlet lines for salt and Silver, a temperature probe, the plated Silver billet, and the bridge. The bridge is a mockup in this but not in the other picture. The bridge is connected to the salt solution beaker on the far right which contains the reference electrode.
The pump is partially seen to the far left.
Both electrodes are connected to the VOM. It is then connected to a PC with a cable and data logging can be carried out as a function of time.
PE
I am now at the point of being able to replicate results to within about +/- 5 mv, but with a slope adjustment as the solution becomes more dilute. I am working on that. It may be due to having immersed too little of the billet in the electrolyte.
Here are photos of the reference electrode, the billet, the bridge and a trial setup of a beaker ready for precipitation of a real emulsion. The reference electrode is immersed in its conditioning/storage solution, and the bridge is also immersed in its own gel/salt storage solution. The bridge is marked as to polarity and the salt that it is to be used with. These are two critical items in this measurement.
The making beaker in the 4th picture contains 2 inlet lines for salt and Silver, a temperature probe, the plated Silver billet, and the bridge. The bridge is a mockup in this but not in the other picture. The bridge is connected to the salt solution beaker on the far right which contains the reference electrode.
The pump is partially seen to the far left.
Both electrodes are connected to the VOM. It is then connected to a PC with a cable and data logging can be carried out as a function of time.
PE
Attachments
In the first photo - the one of the reference electrode - it looks like you have the ref electrode in it's storage tube, right?
I like how you can see the coating/plating on the silver electrode, that's good to know as it should help determine how much plating has occured.
The last photo is getting pretty crowded with tubes... Make sure you leave room for the stir bar! ;^)
Also, you're not seeing any interference from using a magnetic stirrer, right?
I like how you can see the coating/plating on the silver electrode, that's good to know as it should help determine how much plating has occured.
The last photo is getting pretty crowded with tubes... Make sure you leave room for the stir bar! ;^)
Also, you're not seeing any interference from using a magnetic stirrer, right?
Yes, I keep my reference electrode in the storage solution at all times. I actually store the Silver electrode in distilled water, and the bridge is stored in electrolyte gel.
I could not get the stir bar in. Actually, I may need a prop mixer.
Actually, I may need a prop mixer.
There has been no apparent effect from magnetic stirring at all. That does not mean that I will never see it, but so far nothing. If there were to be an effect, i guess I would go to shielded cables.
PE
I could not get the stir bar in.
 Actually, I may need a prop mixer.
Actually, I may need a prop mixer.There has been no apparent effect from magnetic stirring at all. That does not mean that I will never see it, but so far nothing. If there were to be an effect, i guess I would go to shielded cables.
PE
I have gotten several comments both positive and negative about this work. Some want to know why I am posting such complex technical material and I tried to explain that earlier. This methodology is the gateway to controlled, modern, high speed emulsion making.
But, I have overlooked the other use which is also very important but has a much more immediate impact.
Measuring the vAg of an emulsion during the wash step will help you tell whether the wash has been carried out to the optimum point. By measuring the vAg, you can tell how much halide is left in the emulsion and whether the wash is complete, incomplete or overdone. If overdone, it tells you how much extra salt should be added to bring the emulsion to the right salt level for best keeping and best speed. Also, at this point, you do not need a bridge.
I need not comment on those who have responded in a positive manner except to say thanks.
PE
But, I have overlooked the other use which is also very important but has a much more immediate impact.
Measuring the vAg of an emulsion during the wash step will help you tell whether the wash has been carried out to the optimum point. By measuring the vAg, you can tell how much halide is left in the emulsion and whether the wash is complete, incomplete or overdone. If overdone, it tells you how much extra salt should be added to bring the emulsion to the right salt level for best keeping and best speed. Also, at this point, you do not need a bridge.
I need not comment on those who have responded in a positive manner except to say thanks.
PE
rmazzullo
Subscriber
I have been waiting for this aspect of emulsion making to be discussed for quite a while, and am thrilled that process control on a small scale is that much closer to reality. Repeatable, custom tailored emulsions are within reach.
Once subbing polyester is nailed down, things could get really interesting.
Bob M.
Once subbing polyester is nailed down, things could get really interesting.
Bob M.
" Some want to know why I am posting such complex technical material ....."
PE and All,
Tell them to take up kniting. Nice and simple and won't get their hand dirty.
Or better yet, just watch the TV.
Realy, this is not a vocation or avocation for those who cannot see Beauty in the Complex.
Bill
PE and All,
Tell them to take up kniting. Nice and simple and won't get their hand dirty.
Or better yet, just watch the TV.
Realy, this is not a vocation or avocation for those who cannot see Beauty in the Complex.
Bill
Bill;
Rather a strong reaction, but these were polite inquiries and I answered as such. There is no problem but I felt that more information was needed. Thanks.
PE
Rather a strong reaction, but these were polite inquiries and I answered as such. There is no problem but I felt that more information was needed. Thanks.
PE
Yes PE,
I do get carried away sometimes. No real insult intended.
Bill
I do get carried away sometimes. No real insult intended.
Bill
Making electrodes and measuring vAg off hand seems rather simple, although in practice it's taken a bit more work than expected! It's the making the rest of the emulsion and coating that seems to me to be the more complex task...
The measurement of vAg presented a lot of unexpected difficulties. This work compared to emulsion making is on the same order of unexpected difficulties in that every emulsion I make that is new, is different than I expect compared to what I might have gotten or seen made at EK. But, each of these tasks, vAg measurement and actual making present problems that, once solved, are solved and explainable. The same goes for coating.
I might add that those here that have claimed to be able to measure vAg have me puzzled, as they claim it to be easy. I knew how to do it, and still found it relatively hard to get to this point. Now, it will be easy for everyone else.
PE
I might add that those here that have claimed to be able to measure vAg have me puzzled, as they claim it to be easy. I knew how to do it, and still found it relatively hard to get to this point. Now, it will be easy for everyone else.
PE
Experiments nearly complete
I am nearing the end of the experiments to establish an easy and inexpensive method of measuring vAg during emulsion making.
I now have 3 Silver electrodes to be used for Chloride, Bromide and Iodide. They were all plated for 4 minutes using 2 AA batteries. They must be kept and used under reduced light. These electrodes, once plated, will keep for at least 1 week in distilled water. There is a tiny drift of about 2 mv during that time, but some have kept better than others.
I have 3 bridges with polarity and salt marked on them. They seem to keep as long as the ends are wetted with electrolyte gel.
Measurements seem to fall within +/- 4 mv of the correct values from 0.1 molar to 2.0 molar salt for all three halides. There is a slope value associated with this, so that at one end of the concentration scale, the error may be +4 mv and at the other end of the concentration scale it might be -4 mv. I am working on a slope control. I have also built a zero controller in a black box, so that the actual and calculated results can be made to coincide if there is an offset. This is similar to zeroing a pH meter.
I have devised a plater for short and long electrodes (1" - 6" or 2.5 cm to about 15 cm).
I have tested the electrode setup from 100 ml (about 3 cm) up through nearly the full length of the long electrodes along with full and partial plating and have gotten similar results. However, solution level must never go above the plating level of any electrode for stable results.
My next experiments involve electrode length (is 6" best or will 3" do?) and plating salt (do I need a halide or can I use Sulfide?).
In any event, when finished, this entire setup will cost under $150 USD. And, using the meter I suggest, it allows for data logging, but not control. I now have the data logger working perfectly with a 5 meter (~15 ft) cable. This allows the computer to be out of range of potential splashes.
I have verified this data with the Radio Shack meter mentioned above, with an el-cheapo analog RS meter, a Heath meter and a very very sensitive Keithly meter capable of very high accuracy. All of the data seems to be in agreement so I am ready to go on and finish this.
The one major problem remaining is that the plating seems uneven no matter what I do, and I think that this is part of the reason that my data is slightly off. Our electrodes at EK were very smooth and even, but mine here have spots of light and dark that develop during the plating operation.
If anyone is interested, I would be happy to make a custom Silver electrode for them. The cost would probably be in the range of $100 or less. This inclues a 6" or 3" Silver electrode sealed in glass with a 3' (1M) cable with a gold plated banana plug. The electrode is #12 Silver wire 99.99% pure. Usable length is about 5" or 1".
Pictures of this all will follow soon.
PE
I am nearing the end of the experiments to establish an easy and inexpensive method of measuring vAg during emulsion making.
I now have 3 Silver electrodes to be used for Chloride, Bromide and Iodide. They were all plated for 4 minutes using 2 AA batteries. They must be kept and used under reduced light. These electrodes, once plated, will keep for at least 1 week in distilled water. There is a tiny drift of about 2 mv during that time, but some have kept better than others.
I have 3 bridges with polarity and salt marked on them. They seem to keep as long as the ends are wetted with electrolyte gel.
Measurements seem to fall within +/- 4 mv of the correct values from 0.1 molar to 2.0 molar salt for all three halides. There is a slope value associated with this, so that at one end of the concentration scale, the error may be +4 mv and at the other end of the concentration scale it might be -4 mv. I am working on a slope control. I have also built a zero controller in a black box, so that the actual and calculated results can be made to coincide if there is an offset. This is similar to zeroing a pH meter.
I have devised a plater for short and long electrodes (1" - 6" or 2.5 cm to about 15 cm).
I have tested the electrode setup from 100 ml (about 3 cm) up through nearly the full length of the long electrodes along with full and partial plating and have gotten similar results. However, solution level must never go above the plating level of any electrode for stable results.
My next experiments involve electrode length (is 6" best or will 3" do?) and plating salt (do I need a halide or can I use Sulfide?).
In any event, when finished, this entire setup will cost under $150 USD. And, using the meter I suggest, it allows for data logging, but not control. I now have the data logger working perfectly with a 5 meter (~15 ft) cable. This allows the computer to be out of range of potential splashes.
I have verified this data with the Radio Shack meter mentioned above, with an el-cheapo analog RS meter, a Heath meter and a very very sensitive Keithly meter capable of very high accuracy. All of the data seems to be in agreement so I am ready to go on and finish this.
The one major problem remaining is that the plating seems uneven no matter what I do, and I think that this is part of the reason that my data is slightly off. Our electrodes at EK were very smooth and even, but mine here have spots of light and dark that develop during the plating operation.
If anyone is interested, I would be happy to make a custom Silver electrode for them. The cost would probably be in the range of $100 or less. This inclues a 6" or 3" Silver electrode sealed in glass with a 3' (1M) cable with a gold plated banana plug. The electrode is #12 Silver wire 99.99% pure. Usable length is about 5" or 1".
Pictures of this all will follow soon.
PE
I was thinking about your silver electrode and how you sealed it... are you using glass at both ends?
How do you seal the plastic coated cable end?
Ray
How do you seal the plastic coated cable end?
Ray
The glass tube is sealed with epoxy with the cable to Silver junction inside of the tubing. That is the reason for the sealed glass, to protect the junction from corrosion and metal contamination. No moisture should enter the tube nor should it touch the Silver to wire junction. The other end is self sealed in the banana plug shround, and is 3' away from any contamination.
PE
PE
Ray - one the silver electrode I made for I took a couple inches of the 12 ga., 99.99% silver wire mentioned above and soldered it to the lead wire of a shielded video cable with a BNC connector on the other end. (The cable is from Radio Shack - I simple cut one end of the cable and set aside the unused half). I mounted it inside a 6mm diameter, 6 inch long pyrex glass tube by stripping back some of the shielding and the outer part of the video cable and slid the end with the silver wire soldered on to it into the pyrex tube.
I had hoped to have the shielding on the cable all they way to just a bit above the solder joint, but a 6 mm tube was too small in the inside diameter for it to fit. By removing some of the shielding, I was able to slide the silver wire and the teflon insulation around the lead wire into the glass tube.
I plan on getting some larger glass tubing - either 8 or 9 mm diameter so I can get the BNC video cable inside without having to remove the shielding, or perhaps 12 mm diameter so it's the same diameter as a pH meter so it fits into a standard pH electrode stand. Also, instead of cutting the 6 foot BNC cable in half, I'll simply snip on BNC connector off so I can use the full 6 ft of lead on the cable so the pH meter is not so close to the emulsion setup.
Note that I use a pH meter in millivolt mode rather than a standard VOM volt meter like PE so I prefer the BNC connector over a bannana connector, but adaptors can be used to mate up one with the other if needed.
With the silver wire sticking out one end of the glass tube, and the lead wire out the other end of the glass tube, I took some 5 minute epoxy that I prepared and used a pipette to squirt some of the epoxy into both ends of the glass tube. This sealed both ends. I wiped off any epoxy that was on the exposed end of the silver wire. There was about 1.5 inches of silver outside the glass tube, and about 0.5 inches inside.
I had hoped to have the shielding on the cable all they way to just a bit above the solder joint, but a 6 mm tube was too small in the inside diameter for it to fit. By removing some of the shielding, I was able to slide the silver wire and the teflon insulation around the lead wire into the glass tube.
I plan on getting some larger glass tubing - either 8 or 9 mm diameter so I can get the BNC video cable inside without having to remove the shielding, or perhaps 12 mm diameter so it's the same diameter as a pH meter so it fits into a standard pH electrode stand. Also, instead of cutting the 6 foot BNC cable in half, I'll simply snip on BNC connector off so I can use the full 6 ft of lead on the cable so the pH meter is not so close to the emulsion setup.
Note that I use a pH meter in millivolt mode rather than a standard VOM volt meter like PE so I prefer the BNC connector over a bannana connector, but adaptors can be used to mate up one with the other if needed.
With the silver wire sticking out one end of the glass tube, and the lead wire out the other end of the glass tube, I took some 5 minute epoxy that I prepared and used a pipette to squirt some of the epoxy into both ends of the glass tube. This sealed both ends. I wiped off any epoxy that was on the exposed end of the silver wire. There was about 1.5 inches of silver outside the glass tube, and about 0.5 inches inside.
By removing some of the shielding, I was able to slide the silver wire and the teflon insulation around the lead wire into the glass tube. [snip]
Note that I use a pH meter in millivolt mode... so I prefer the BNC connector over a bannana connector, but adaptors can be used to mate up one with the other if needed. [snip]
I took some 5 minute epoxy that I prepared....
Thanks Kirk for that very good answer.
I think I am using the adaptor...
Two ?s went through my head though...
a)
Teflon insulation on Radio Shack cable?
b)
5min=5 min to set ?
You're using a double solution epoxy?
Does it ruin your pipette? (or are you using throw-a-ways?)
Last edited by a moderator:
The glass tube is sealed with epoxy....
PE
I see. Somehow I thought you were heat sealing it... I was not too successfull with that approach.

It's "5 minute epoxy" which two part epoxy that sets in 5 minutes.
Yeah, I thought about heat-sealing the silver into the glass, and that's just too complicated. And a lot of pH electrodes have epoxy bodies and I figured why not try the 5 minute type for sealing the tube. It seems to work and it doesn't appear to corrode the silver wire - at least the stuff I tried.
Maybe the inner dielectric insulator is polyethylene instead of teflon. I'd have to look at it again and maybe burn some to see if it gives off really acidic fumes - it's a really easy way to tell polyethylene from teflon.
I used little 3 ml polyethylene disposable pipettes to transfer the epoxy. I didn't want to use glass as I didn't want to break the tip of the glass pipette into the epoxy.
Yeah, I thought about heat-sealing the silver into the glass, and that's just too complicated. And a lot of pH electrodes have epoxy bodies and I figured why not try the 5 minute type for sealing the tube. It seems to work and it doesn't appear to corrode the silver wire - at least the stuff I tried.
Maybe the inner dielectric insulator is polyethylene instead of teflon. I'd have to look at it again and maybe burn some to see if it gives off really acidic fumes - it's a really easy way to tell polyethylene from teflon.
I used little 3 ml polyethylene disposable pipettes to transfer the epoxy. I didn't want to use glass as I didn't want to break the tip of the glass pipette into the epoxy.
Last edited by a moderator:
I am also saving my 120 paper backing. I have a drawer full of them.
PE
I did that for a while! Finally had to recycle them. One has a limited amount of space. :-(
Ed
I did that for a while! Finally had to recycle them. One has a limited amount of space. :-(
Ed
Yeah Ed. See the other thread on 120!
I still have my drawer of paper backing.
PE
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |