@ Marco: Many thanks for your input, I really appreciate it.
These 2g/l seem to come all from the same original source, but I have not been able to locate / read the publication by Karl Frank.
It would be good to properly analyse the assumptions and conditions under which this figure was originally published - and to what extent experimental testing and further research has been made.
One of the assumptions stated relates to Lith Film researched and not to a Lith effect on conventional photographic paper. Furthermore, I´d guess that a main design criterion was reproducibility at industrial standard, i.e. put film and developer in a black box and after a pre-set period of time take a finished product out, no inspection, snatch point or something similar. Furthermore, I´d guess that short development times were a requirement, too, and maybe the developer was also designed as one-shot product anyway?
Under the a.m. mentioned conditions (highly reactive, short development time to get to Dmax, one shot product, short tray live only), it makes sense to use a minimum sulphite HQ developer, i.e. nothing or almost nothing.
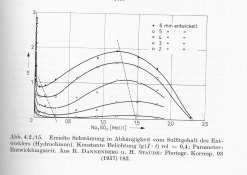
I have found some older research showing the relationship between Dmax and Sulphite content, clearly stating that after a steep decline in Dmax while slowly increasing Sulphite concentration, after a certain point the Dmax actually increases again together with the Sulphite before the developer eventually dies at a very high sulphite concentration. I will try to scan the graph and share tonight or tomorrow.