I realize this is an old thread, but let me continue.
For a long time I have been wondering about diluting C-41 developer. I just found a copy of Kodak's document on using C-41 chemicals. (Someone here at photrio provided a link to the document.) From the data in that document I was able to make some calculations to project the results for diluted C-41 developer at a 50% dilution. After I did the calculations I found this thread, which is very relevant to my question and calculations. I also found another thread on the topic of C-41 developer dilution. Here is the link.
https://www.photrio.com/forum/threads/diluted-c41-tested.175919/. I could only reply to one thread, so I am choosing the earlier one.
Let me start with the bottom line. According to my calculations if you do a 50% dilution of the standard C-41 developer (LORR version), and leave out both the starter and the part B of the developer it should produce negatives that are very close to negatives produced under standard C-41 conditions.
Based on my calculations (which I will explain in more detail below) here is what you would do. Mix LORR C-41 developer but omit part B of the developer. Dilute the developer to the normal working concentration. Do not add the developer starter solution. Dilute again with an equal part of water. (There is a subtlety regarding this dilution that I might discuss later in the post, or maybe not, but I don't think that subtlety is very important, so I will skip it, at least for now.)
What you have now is a developer at 50% of the normal working concentration, but it is missing both part B of the developer and the developer starter. Omitting those to solutions basically compensates for the fact that this developer is not a replenished developer.
Use the developer as if it were a normal replenished developer, i.e. at normal times and temperatures.
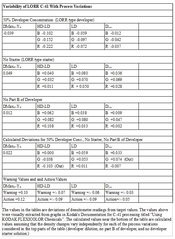
Based on my calculations here are the what the deviations in sensitometer readings would be compared to the target values
In the table above I retained all of the decimal places I used for the calculation, but you can round them off to two decimal places. Most of the values are within the specified ranges. Two of them are out of range, but only slightly out of range. The control limit for
I am attaching the files from which I took values for the calculation and the calculated results. The values were extracted visually from the graphs. I included three decimal points in the extracted data, which is probably within plus or minus 0.002 of the "true" values one would get from a perfect extraction of the values from the graphs.
With only two exceptions, all of the values are within control limits. The HD-LD parameter is -0.09, but the predicted value I get for this witches brew is -0.103 for the Red densitometer channel, which is out-of-control. However, this is so close that I doubt if anyone would be able to tell the difference in practice between an in-control process at an HD-LD of -0.09 and an out-of-control process at an HD-LD of -0.103. The green channel is out of control for Dmin, but only by +0.024, which again is close enough that I don't think it would make a difference that anyone would notice. (Note: Dmin is in the extreme shadow range.) And even if it did make a difference it is highly likely that any difference could be fixed by a very minor tweak in the curves in a scanned image or a very slight change in filtration in a darkroom-printed image.
I have not tried to verify these calculations using experimental measurements. There are a few limitations to this theoretical study. The first is the accuracy of Kodak's data. We just don't know how accurate the curves are.
The second limitation is the accuracy with which I extracted the numbers from the graphs. I already gave an estimate of what the precision is for the number-extraction process (probably plus or minus 0.002 units).
The most significant limitation is the underlying assumption made in the calculations, which is that the process variations affect the densities in an independent manner, so they can be combined by simply adding the variations together. For example, if the altered dilution gives a change in Dmin of value X, omitting part B of developer gives a change in Dmin of Y, and omitting the starter solution gives a change in Dmin of Z, then does the combination of process changes change the Dmin by X+Y+Z? Probably not, but I'll bet the assumption is probably at least roughly correct, and if that is true then the conclusion is valid that the altered C-41 process is good enough from all practical purposes.
Also to be considered is that this scheme is intended to be used with fresh solutions in a one-shot process, so any additional variability that can enter into the development process in a replenished system are eliminated, and that has to count for something.
Everyone is probably wondering about eliminating part B from the developer. Looking at the curves in the attached files it's pretty clear the part B mainly functions as a restrainer. Eliminating it is a major factor in bringing the activity of the dilute developer up to that of the developer at standard concentration.
Eliminating the starter is also a factor in bringing the performance of the diluted developer close to that of the developer under standard conditions.
Of course it's fair to ask whether anyone cares. Specifically, why would anyone want to run an altered C-41 process with diluted developer? I will leave that question for a separate discussion, but feel free to discuss the issue if you want.