You might be tempted to use Dust-Off (or a clone of it) to drive the oxygen out of a partially used bottle of XTOL. Well, I found the gas dissolves into XTOL and makes the XTOL more active. Here are the curves:
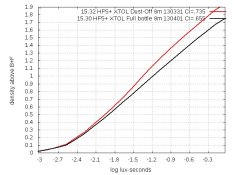
The bottle was 3/4 full, so only 1/4 was Dust-Off gas. XTOL's pH is unchanged, so Dust-Off is doing something else to the chemistry. And it's probably not what you want. The clone I use says it contains difluoroethane. Rudeofus warned me months ago that this is not inert. I should have heeded his warning.
Mark Overton

The bottle was 3/4 full, so only 1/4 was Dust-Off gas. XTOL's pH is unchanged, so Dust-Off is doing something else to the chemistry. And it's probably not what you want. The clone I use says it contains difluoroethane. Rudeofus warned me months ago that this is not inert. I should have heeded his warning.
Mark Overton



 . Marks entry is most welcome and when finally refined will be a delight and comfortable alternative to Kodak's if only for the smaller amount of concentrate needed in-house.
. Marks entry is most welcome and when finally refined will be a delight and comfortable alternative to Kodak's if only for the smaller amount of concentrate needed in-house.
