As is adaptation.
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
D-76: full strength or 1:1?
-
A
- Thread starter paulfish4570
- Start date
Recent Classifieds
-
For Sale Contax TLA 200 Flash for G Rangefinder - New Old Stock
- Started by davela
-
Want to Buy Beseler 23C Lens Board Spring . . .
- Started by Locutus of Borg
-
For Sale Widelux Accessory Sweater $210 with shipping to US address
- Started by laser
-
For Sale Durst M805 Color Complete Darkroom Enlarger Kit (B&W & Color)
- Started by Marketa
-
For Sale Silvergrain Classics magazines #21,22,23,24 in like new condition
- Started by Gaston 012
Forum statistics
Why add and multiply?:confused:I am. I stick to 1+x!
Whenever two systems exist to do an identical thing, confusion is pre-programmed. Context is important but consistency is key.
I don't see why all the confusion. They taught us this sort of stuff in middle school. There is a difference between ratio and proportion.
- Ratios are expressed as x:y and are read as "x to y". Example: 1 part stock solution to 3 parts water (1:3). This often results in 4 parts final solution, though not always. It depends on the liquids being mixed.
- Proportions are expressed as fractions and are read as "x in y". Example: 1 part stock solution in 4 parts final volume diluted with water (1/4 strength).
Edit: The more I think about it, the more I think that I may have my terms wrong...getting old is hell...
Another Edit: This is a real can of worms. I just got done reading the Wikipedia article and while ratio has a general meaning as the one I use, when applied to dilutions, it depends on whether you are talking about dilution factor or dilution ratio. Both use the same notation. Certain disciplines (e.g. research chemistry and biology) use dilution factor which allows for easy calculation of concentration. The section on dilution ends with this strange sentence:
In other areas of science such as pharmacy, and in non-scientific examples, dilutions normally given as a plain ratio of solvent to solute.
Go figure...
Steve
Last edited by a moderator:
Tony-S
Subscriber
1:1 is the same as undiluted. It helps to preserve the math of making dilutions. For example, to make 1000 ml of a 1:4 dilution, take 1000/4 = 250 ml of stock solution. To determine the volume of water, simply subtract this value from your needed volume: 1000 - 250 = 750. Thus 250 ml of stock solution (e.g., D-76) plus 750 ml of water is 1000 ml at 1:4.
Using the "+" system this is 250+750, or reduced it's 1+3.
Using the "+" system this is 250+750, or reduced it's 1+3.
Last edited by a moderator:
Why add and multiply?:confused:
Because we buy gasoline in liters (or litres) and calculate fuel consumption in miles/gallon?
BTW...
So what if the directions say to mix chemicals A, B, and C in the ratio 1:2:3?

Steve
So what if the directions say to mix chemicals A, B, and C in the ratio 1:2:3?

Steve
Tony-S
Subscriber
Tell them they need better instructions.
- Joined
- Mar 2, 2008
- Messages
- 5
- Format
- 4x5 Format
I shoot 4X5 and vary development to change highlights. To do this I dilute 1/3 developer to 2/3 water. Longer development times are the result. My normal development time is 14 minutes. This inconvenience is trumped by control over highlights. Most exposures with the sun shinning will be over exposed and cutting development time in combination with diluted developer is a good way to prevent to much silver build up on the negative. Diluted developer, gentle agitation, and shorter development times give thinner appearing negatives which will let your paper have a broad tonal range. Its the print that counts in this situation the negative is the means to get there. Most of my actual development times are around 8 minutes when i have a scene that is 3-4 stops beyond the range of my film. Cutting development times gives the tonal range I want in my negatives. A good rule of thumb if you try this is to not have dark/black in the highlights of your negatives.
"light-tight Paterson funnel does NOT lock in the normal position with spout down in the small Super System 4 tank, which I should have checked before going dark."
The cores for the Super System 4 and the regualr system 4 are slightly different. you have to use the right one for the tank.
The cores for the Super System 4 and the regualr system 4 are slightly different. you have to use the right one for the tank.
I like D-76 any which way you pour it. I'd suggest 1:1 or greater dilution if you want to use it as a one shot developer, and straight if you want to re use or replenish.
This thread has provided an education; glad I joined this forum ... 

I like D-76 any which way you pour it. I'd suggest 1:1 or greater dilution if you want to use it as a one shot developer, and straight if you want to re use or replenish.
I like to second this statement. For simplicity and consistency I like to use it 1+1, one-shot.
I started use D-76 full strengh and then switch to 1+1. I still like it!
Jeff
Jeff
Thomas Bertilsson
Member
This is correct. 1:1 = stock solution. Dilution factor = 1 = 1(solute, developer) + 0(solvent, water)
Quote:
1. Simple Dilution (Dilution Factor Method based on ratios)
A simple dilution is one in which a unit volume of a liquid material of interest is combined with an appropriate volume of a solvent liquid to achieve the desired concentration. The dilution factor is the total number of unit volumes in which your material will be dissolved. The diluted material must then be thoroughly mixed to achieve the true dilution. For example, a 1:5 dilution (verbalize as "1 to 5" dilution) entails combining 1 unit volume of solute (the material to be diluted) + 4 unit volumes of the solvent medium (hence, 1 + 4 = 5 = dilution factor). The dilution factor is frequently expressed using exponents: 1:5 would be 5e-1; 1:100 would be 10e-2, and so on.
Example 1: Frozen orange juice concentrate is usually diluted with 4 additional cans of cold water (the dilution solvent) giving a dilution factor of 5, i.e., the orange concentrate represents one unit volume to which you have added 4 more cans (same unit volumes) of water. So the orange concentrate is now distributed through 5 unit volumes. This would be called a 1:5 dilution, and the OJ is now 1/5 as concentrated as it was originally. So, in a simple dilution, add one less unit volume of solvent than the desired dilution factor value.
End Quote.
Source: Bates College Website
Quote:
1. Simple Dilution (Dilution Factor Method based on ratios)
A simple dilution is one in which a unit volume of a liquid material of interest is combined with an appropriate volume of a solvent liquid to achieve the desired concentration. The dilution factor is the total number of unit volumes in which your material will be dissolved. The diluted material must then be thoroughly mixed to achieve the true dilution. For example, a 1:5 dilution (verbalize as "1 to 5" dilution) entails combining 1 unit volume of solute (the material to be diluted) + 4 unit volumes of the solvent medium (hence, 1 + 4 = 5 = dilution factor). The dilution factor is frequently expressed using exponents: 1:5 would be 5e-1; 1:100 would be 10e-2, and so on.
Example 1: Frozen orange juice concentrate is usually diluted with 4 additional cans of cold water (the dilution solvent) giving a dilution factor of 5, i.e., the orange concentrate represents one unit volume to which you have added 4 more cans (same unit volumes) of water. So the orange concentrate is now distributed through 5 unit volumes. This would be called a 1:5 dilution, and the OJ is now 1/5 as concentrated as it was originally. So, in a simple dilution, add one less unit volume of solvent than the desired dilution factor value.
End Quote.
Source: Bates College Website
1:1 is the same as undiluted. It helps to preserve the math of making dilutions. For example, to make 1000 ml of a 1:4 dilution, take 1000/4 = 250 ml of stock solution. To determine the volume of water, simply subtract this value from your needed volume: 1000 - 250 = 750. Thus 250 ml of stock solution (e.g., D-76) plus 750 ml of water is 1000 ml at 1:4.
Using the "+" system this is 250+750, or reduced it's 1+3.
This is correct. 1:1 = stock solution. Dilution factor = 1 = 1(solute, developer) + 0(solvent, water)...[/URL]
Thomas
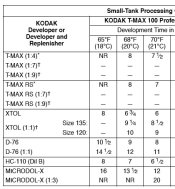
I see it that way too, but unfortunately, that's not the way Kodak sees it. See attached clip from their tech-pub F32, listing D76 and D76 1:1 separately, so apparently, not the same thing to them.
Attachments
I'm a little confused,all ways thought that 1:3 meant 1 part solution to 3parts water (4 prts total) am I wrong.:confused:Have no chemistry background.
Mike
That is correct - chemically, and mathematically. Though there may be other interpretations in a different context. In chemistry and mathematics, the following are equivalent:
A:B
kA:kB where k is any nonzero constant
A/(A+B):B/(A+B) (This gives the amounts when the whole quantity is 1 unit)
BTW, I always get thrown off reading the label on Ilford Rapid Fixer. The label's top gives capacity for 1+4, and if you peel back to read instructions it says 1+3.
Thomas
I see it that way too, but unfortunately, that's not the way Kodak sees it. See attached clip from their tech-pub F32, listing D76 and D76 1:1 separately, so apparently, not the same thing to them.
Unfortunately Kodak have used both ways over the years which is rather misleading and so they are technically wrong. In older publications they were much more precise and correct specifying 1 part D76 + 1 part water etc.
1:10 means 1 part made up to 10, Ilford make it clearer by saying 1+9, 1+19 etc. In science and medicine 1:100 means dilute 1 part to 100 total, so there's no such thing as 1:1, its FS.
Ian
Originally Posted by mike c
I'm a little confused,all ways thought that 1:3 meant 1 part solution to 3parts water (4 prts total) am I wrong.Have no chemistry background.
Mike
Calling it 1+3 would solve this confusion for me.
I'm a little confused,all ways thought that 1:3 meant 1 part solution to 3parts water (4 prts total) am I wrong.Have no chemistry background.
Mike
That is correct - chemically, and mathematically. Though there may be other interpretations in a different context. ...
Calling it 1+3 would solve this confusion for me.
Thomas Bertilsson
Member
Calling it 1+3 would solve this confusion for me.
True. And, :rolleyes:, it's consistent...
Whoudda thunk mixing developers was so complicated?
Using something like HC-110 or Rodinal also helps, because they are usually diluted so much it doesn't matter if you mix 49 or 50 parts water to one part developer.
I had forgotten how much fun this little 'problem' could be. So instead I use replenished Xtol, which starts with stock and is replenished with stock.
To the Original Poster of this thread. Make a simple test where you sacrifice a few hours of your life to find out for yourself.
The proof really is in how well the negatives developed in either stock, or 1pt D76 stock + 1pt water, will print - in your darkroom (with your enlarger, your water source, your print developer, your paper of choice, your camera, your light meter, your light metering technique, etc....).
That is correct - chemically, and mathematically. Though there may be other interpretations in a different context. In chemistry and mathematics, the following are equivalent:
The confusion comes from a very simple detail that seems to elude most posters -- ratios without stated units are meaningless. Different contexts have different common usage, and within one of those contexts,you are safe. Otherwise, you're just hoping for the best.
Kodak's convention is stock:water, some use stock:final_volume, and there are others possible, especially when you get past discussing dilutions.
My D76 goes to 11.

The confusion comes from a very simple detail that seems to elude most posters -- ratios without stated units are meaningless. Different contexts have different common usage, and within one of those contexts,you are safe. Otherwise, you're just hoping for the best.
Kodak's convention is stock:water, some use stock:final_volume, and there are others possible, especially when you get past discussing dilutions.
My D76 goes to 11.
Very good point!
I wish Kodak would clearly state this somewhere. But again, the 1+x convention is less confusing, because it does not require this distinction.
Actually, perhaps because electronics and engineering run in my background, having a situation where one part of concentrate and one part solvent yield 2 parts of a solution, could be expressed as 1->2, so that typical Rodinal dilutions would be 1.-51 or 1->26.
having the instructions say to mix with water 1:1 is rather obvious. with the units being irrelevant to the result. - one scoop of D.76, one scoop of water. Apperently some of the chemistry folks have stolen the notation and re-defined it to something that non-chemists would find confusing. 1:3 being interpreted to mean for example that 250ml of concentrate is added to a TC measure and water is added to the 750Ml mark. to an engineer 1:3 means that the 250ml of concentrate is added to 750ml of water, with the final volume assumed to be close to 1l (depending if the concentrate dissolves in water or not.)
having the instructions say to mix with water 1:1 is rather obvious. with the units being irrelevant to the result. - one scoop of D.76, one scoop of water. Apperently some of the chemistry folks have stolen the notation and re-defined it to something that non-chemists would find confusing. 1:3 being interpreted to mean for example that 250ml of concentrate is added to a TC measure and water is added to the 750Ml mark. to an engineer 1:3 means that the 250ml of concentrate is added to 750ml of water, with the final volume assumed to be close to 1l (depending if the concentrate dissolves in water or not.)
... to an engineer 1:3 means that the 250ml of concentrate is added to 750ml of water, with the final volume assumed to be close to 1l. ...
To this engineer 1:3 means 1/3 concentrate.
To this engineer 1:3 means 1/3 concentrate.
e.g. 100ml concentrate + 200ml water?
Tom
Not being either a chemist or engineer I guess is an advantage. In 50 years of using Kodak and a few other brands, I never had a problem with the instructions. Perhaps, just dumb luck on my part.
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |