I am deeply interested, but do not really have the mathematical skills to comprehend it very well!
One of the tough things about the subject is that theoretical descriptions are full of assumptions and simplifications, just to make the problem tractable. On the other hand, it leaves plenty of room for experimental discoveries.

Actually, there HAVE been several related photographic procedures that seem to use this principal.
I have a small file on this method of color photography as I am planning some experiments in that area... I don't think I was aware of the Science article...
.........
What is your interest in this subject?
(if you do not mind my asking)
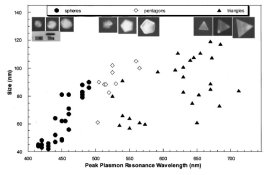
My interest is partly professional: I work in a physics department looking at the links between the electronic properties of nanostructures and their physical properties, particularly friction. The electronic structure is what determines the optical response in the visible region and there are a whole raft of reasons why I have been trying to at least keep up with the literature. Mostly I want to use simple optical microscopy to characterise a dispersion of different nanoparticles, rather than having to resort to more complex and finicky electron or probe microscopes; but also because the optoelectronics are an essential part of any applications of my work, and so much of the froth around funding and status in science departments these days revolves around patents and commercial spinoffs.
Also, I think the subject's cool

Also, I would like to do ULF colour, and I can't see myself ever affording my favourite colour emulsions in 12x15 or 15x15 size.
All the photographic work I have seen has been with AgCl emulsions. There was a lot of excitement in the early days of spectral recording onto AgCl on bare paper when people often observed colours, which in some cases seemed in direct correspondence with the colour of the light from the relevant part of the spectrum.
Bensley's method is a sort of bichromic one that builds on this. He resensitized a positive print made from the orange-red part of the spectrum and re-exposed in register with a negative made with the green-blue part of the spectrum before finally giving a slow, physical development. The existing grains of the positive image provide a template for the growth of AgCl crystals in the resensitisation step, and simultaneously mask the exposure, so they influence the size, shape and position of the silver grains formed in the final step.
It's not mentioned by the references I gave earlier, but once the nanoparticles get within a few diameters of each other their optical fields interact and you get a combination of a colour shift and a much stronger scattering. I'm intrigued by the idea that Bensley has made an all-silver image display colours not so much by tuning the size or shape of the silver grains, but by varying the spacing of the silver grains resulting from the second development. I suspect things are more dull, and he has produced a sort of Retinax pseudo-colour by mixing red and green monochrome images, but it's intriguing enough that I have started to assemble filters and film for some initial tests. The work gets shunted onto the back burner all too often though, so I'd love to hear of anything anyone else tries.








 After seeing Kirk's collection of totally cool used lab equipment, it's almost tempting, and doesn't seem that unreasonable.
After seeing Kirk's collection of totally cool used lab equipment, it's almost tempting, and doesn't seem that unreasonable.