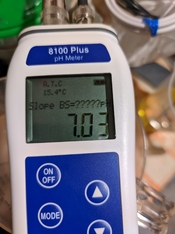
I have a PH meter, but at the moment I have problems with it - it gives a deviation of about +0.3-0.4 and because of the large difference it refuses to calibrate. The electrode is new, but there must be something wrong with the stay or delivery - I will torture it with cleaning fluid, if not - I will order a new one...
Hi, I'm not familiar with that meter, but typically problems with "drifting" are due to the electrodes. In the distant past I used to 1) read a lot of photo chem pH values, and 2) do a lot of troubleshooting of pH electrodes.
That said, I don't know what kind of electrode(s) you are using, but there oughta be some sort of instructions for it/them.
As a very brief primer, there are two electrodes involved. One is the pH electrode, and typically uses a very special glass in the tip; it's thin and delicate, and should not be "messed around" with. The other is called a reference electrode, and... well, let's say that many use a "filling solution" which slowly leaks into the sample, while others use some sort of "gel." If yours uses a filling solution (typically a strongish KCl solution) then you must be sure to keep it adequately filled, and always be sure to keep the filling solution at a higher level than the sample. (If there is a "plug" for the fill hole, remove the plug during use.) Sometimes these can "clog" at the "junction," where the filling solution meets the sample. The electrode manufacturer will typically have instructions on how deal with these things.
Now, many cheaper systems will combine the two into a single physical electrode so it appears that there is only one; but functionally there are two. Where I'm from we would always use separate electrodes (pH vs reference) largely for the ability to troubleshoot by swapping out individual electrodes.
All this aside, here's what I would try, in your position. First, never "wipe" the electrode, but you can blot liquid off the tip, if needed. I note that your photos show a temperature of about 15 C, which is colder than I ever used. Photo chem pH specs are typically given for something like 20 or 25 C; this is where I would be reading samples. Also I would wonder if a "room-temperature" saturated filling solution might be partially precipitated in the junction.
I would try soaking the electrodes (in the manner of taking readings) in a slightly warmer (say 25 C) pH 7 buffer for a couple of hours, then see if this helps. When you change buffers (or samples) I'd lightly rinse down the electrode with distilled water from a plastic squirt bottle, the kind where you squeeze the bottle and water squirts out the nozzle. Then either shake gently or lightly blot to take loose droplets off the tip. When you put it into the next sample, gently swirl the sample container to help mix things up, then let it sit motionless for the reading.
For some more detailed information look at Kodak publication CIS-121. Note that they recommend constant stirring while measuring pH; this is not normally needed for general use. (If you are using a research-grade meter that reads out to 3 decimal places then constant stirring might improve results.)
Many people think it's easy to take pH readings, but there's a lot more to it than meets the eye. Color developers can be especially difficult, with misleading results. If someone wants to be sure they are getting proper results with a C-41 developer I would recommend first mixing an official Kodak mix (as a known-good reference) then take that reading as your future aim.
Best of luck.


 I want to mix photo engineer formula and I got almost all elements for developer. There is one component which is confusing me a bit and I wanted to ask someone who already mix it. It's about Dyethylenetriaminepentaacetic acid pentasodium salt (40% sol.) ----- 8.43g. I am not sure if it's grams or it should be mL , because the formula is given as 40% solution. How did you measured this one?
I want to mix photo engineer formula and I got almost all elements for developer. There is one component which is confusing me a bit and I wanted to ask someone who already mix it. It's about Dyethylenetriaminepentaacetic acid pentasodium salt (40% sol.) ----- 8.43g. I am not sure if it's grams or it should be mL , because the formula is given as 40% solution. How did you measured this one?