Alan Johnson
Subscriber
- Joined
- Nov 16, 2004
- Messages
- 3,308
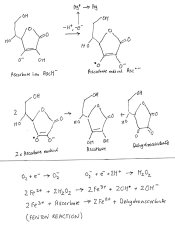
I have been curious to know how Xtol, Ilfosol etc work as details of the chemistry are not published. In 1968 Lee and Miller found regeneration of phenidone by ascorbate in solution appeared to be a simple reduction reaction:
(there was a url link here which no longer exists)
I borrowed from biology publications to make a theory applicable to photography.
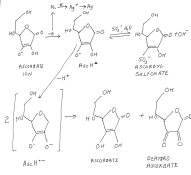
Probably phenidone adsorbed on a grain surface just passes an electron from the ascorbate to a silver ion which is converted to metallic silver.Referring to the attached diagram it is suggested that this electron comes from an ascorbate ion which gives it up to become an ascorbate radical. This radical then converts to ascorbate and dehydroascorbate (called a dismutation reaction), really they should be shown as ions in a correct diagram.
Over a longer period of time than it takes to develop a film, other reactions can occur. The dehydroascorbate further decomposes (reactions not shown).In air oxidation of ascorbate can occur and in some cases is catalysed by metal ions (Fenton reaction).
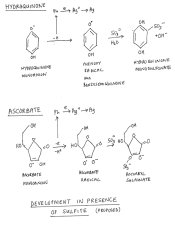
The ascorbate radical has the structure of an ionised semiquinone, cf benzosemiquinone and there may be some similarity with hydroquinone chemistry.I have not considered the effect of sulfite.
(there was a url link here which no longer exists)
I borrowed from biology publications to make a theory applicable to photography.
Probably phenidone adsorbed on a grain surface just passes an electron from the ascorbate to a silver ion which is converted to metallic silver.Referring to the attached diagram it is suggested that this electron comes from an ascorbate ion which gives it up to become an ascorbate radical. This radical then converts to ascorbate and dehydroascorbate (called a dismutation reaction), really they should be shown as ions in a correct diagram.
Over a longer period of time than it takes to develop a film, other reactions can occur. The dehydroascorbate further decomposes (reactions not shown).In air oxidation of ascorbate can occur and in some cases is catalysed by metal ions (Fenton reaction).
The ascorbate radical has the structure of an ionised semiquinone, cf benzosemiquinone and there may be some similarity with hydroquinone chemistry.I have not considered the effect of sulfite.