PhotoChemist
Member
Edit: I missed out a step where you brominate the alcohol and use ammonia before you stick on that sulfone like group.
There doesn't appear to be any total synthesis of this in any literature, found a couple of 2 or 3 step routes that used starting materials you'll never find. The near impossibility to find CD3 in the UK and Europe paired with a bit of boredom had me wonder if synthesis is feasible. I got out a pad of paper and this is what I came up with, CD4 is almost doable but the lithium aluminium hydride, can't imagine that being easy to obtain, there's other ways to do this step but not many ways that will prevent over reaction without finding some difficult to obtain chemicals. This was my best attempt at devising a synthetic route with home synthesis in mind, there are nicer ways to do this but the issue is getting the chemicals, which would be even harder than finding CD3 itself.
So I suppose this is for fun/education only and makes it clear just how this isn't something you're going to do at home, this wouldn't be particularly safe without a fume cupboard, step 4 with mesyl chloride is where things fall apart, it's pretty nasty and I don't think you'd have much chance buying it. May have missed some workup details and haven't covered any purification steps, but I think it would be redundant to bother with it at this point. And like I said it was just for a bit of fun.
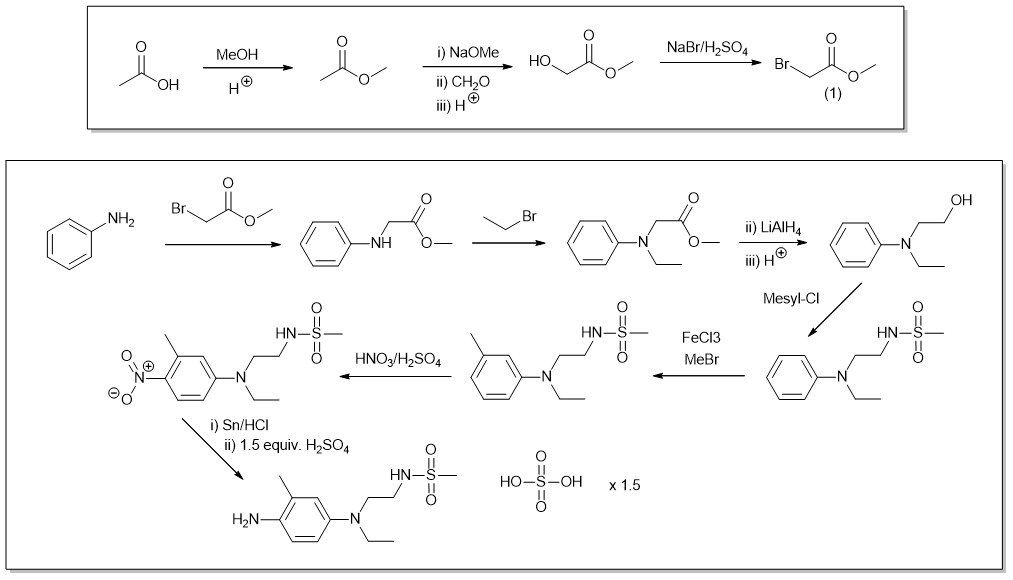
There doesn't appear to be any total synthesis of this in any literature, found a couple of 2 or 3 step routes that used starting materials you'll never find. The near impossibility to find CD3 in the UK and Europe paired with a bit of boredom had me wonder if synthesis is feasible. I got out a pad of paper and this is what I came up with, CD4 is almost doable but the lithium aluminium hydride, can't imagine that being easy to obtain, there's other ways to do this step but not many ways that will prevent over reaction without finding some difficult to obtain chemicals. This was my best attempt at devising a synthetic route with home synthesis in mind, there are nicer ways to do this but the issue is getting the chemicals, which would be even harder than finding CD3 itself.
So I suppose this is for fun/education only and makes it clear just how this isn't something you're going to do at home, this wouldn't be particularly safe without a fume cupboard, step 4 with mesyl chloride is where things fall apart, it's pretty nasty and I don't think you'd have much chance buying it. May have missed some workup details and haven't covered any purification steps, but I think it would be redundant to bother with it at this point. And like I said it was just for a bit of fun.
Last edited:












