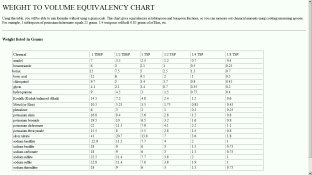
Does anyone know where to find a chart, online, for spoon measurement? Years ago I found one but currently I have had no luck. This, of course, is for volumetric, rather than mass, amounts. Don't want to get into a controversy about how 'inaccurate' such is (there are pros and cons). I know that Anchell's Darkroom Cookbook has one, but I do not have the book. Thank you. - David Lyga
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
spoon measurement
-
A
- Thread starter David Lyga
- Start date
Recent Classifieds
-
For Sale FLM CP34 - L4 II 10X carbon fiber tripod - Excellent Condition
- Started by gphoto120
-
For Sale Mamiya C330 and Mamiya C220
- Started by campy51
-
Want to Buy Canon FTb or EF camera body
- Started by blee1996
-
Want to Buy Replacement green tube for my Aristo VCL8100
- Started by jbrianfoto
-
For Sale Canon AT-1 35mm film camera with two lenses & manual
- Started by J Durr
Forum statistics
Oh my
Boy, the Internet is all over the map on this one. I did find Biblical-era measurements but the only thing I found I could understand on spoons is that a teaspoon is .50 fluid ounces. Good luck. I don't do apps but there should be one on this. I think.
Boy, the Internet is all over the map on this one. I did find Biblical-era measurements but the only thing I found I could understand on spoons is that a teaspoon is .50 fluid ounces. Good luck. I don't do apps but there should be one on this. I think.
Haven't seen much being written about spoon measurement of chemicals in quit some time. Digital scales have become so reasonable that I think most people weight things out.
Regardless of how inaccurate it is I've been using a teaspoon to measure Rodinal for quite awhile now. A teaspoon is supposed to be approximately 4.9ml, I just round it up to 5ml and everything has been going good so far. I develop everything in rodinal 1+50 so for 1 roll of 35mm film in a Paterson tank thats 250ml total which is 5ml or 1 teaspoon of Rodinal.
David,
I've read somewhere that there is really no universally agreed upon definition for teaspoon and tablespoon. I've also read it varies greatly from a brand to a brand of spoons. That said, it is commonly known in US that, teaspoon is 5ml or 5cc, tablespoon is 3 times that at 15ml or 15cc.
I've read somewhere that there is really no universally agreed upon definition for teaspoon and tablespoon. I've also read it varies greatly from a brand to a brand of spoons. That said, it is commonly known in US that, teaspoon is 5ml or 5cc, tablespoon is 3 times that at 15ml or 15cc.
Truzi
Member
- Joined
- Mar 18, 2012
- Messages
- 2,685
- Format
- Multi Format
This probably isn't the kind of answer you want, but why not get a set of measuring spoons and a small graduated cylinder. At least you'll know for your own situation, though it won't help if you are trying to work from someone else's measurements.
You could try some wine-making sites, like:
winemaking.jackkeller.net
It's a long-shot, but you may find something on it.
You could try some wine-making sites, like:
winemaking.jackkeller.net
It's a long-shot, but you may find something on it.
all sorts
There are all sorts of dispensers for small amounts of liquids at your local drug store (Walgreens, etc.) I have a Medicine Dropper and Spoon designed to measure out one or two tablespoons of medication. I guess infants need small doses of medicine so you will find them in the baby department.
There are all sorts of dispensers for small amounts of liquids at your local drug store (Walgreens, etc.) I have a Medicine Dropper and Spoon designed to measure out one or two tablespoons of medication. I guess infants need small doses of medicine so you will find them in the baby department.
- APUGuser19
- Deleted
David,
I've read somewhere that there is really no universally agreed upon definition for teaspoon and tablespoon. I've also read it varies greatly from a brand to a brand of spoons. That said, it is commonly known in US that, teaspoon is 5ml or 5cc, tablespoon is 3 times that at 15ml or 15cc.
I should have elucidated: Long ago I graduated to a graduated cylinder. I merely mentioned 'spoons' because that is all that most know about when it comes to volumetric measurements of raw photo chemicals. I already KNOW that a teaspoon (at least in theory) represents 5ml and a tablespoon represents 15ml.
What I wanted was the g/ml factors for stuff like metol (I think 0.7) and hydroquinone (I think 0.6). There ARE some chemicals that, like water, equate cubic area with mass, such as potassium ferricyanide (i.e., one ml weighs one gram).
It amazed me to find NOTHING on the Internet about this. I merely wanted a conversion table, like in Anchell's Darkroom Cookbook. And, yes, 'Oh My', I also saw the Biblical measurement site also. - David Lyga
- Joined
- Jun 21, 2003
- Messages
- 29,832
- Format
- Hybrid
if / when you find the conversion
can you post it back to this thread?
maybe the moderators can make it a sticky
since there are lots of people who tsp rather than weigh things out...
can you post it back to this thread?
maybe the moderators can make it a sticky
since there are lots of people who tsp rather than weigh things out...
Thank you, Wayne. I have the following for these. I wish to CONFIRM the gram/ml factors:
metol = 0.7
hydroquinone = 0.6
sodium sulfite, anhydrate = 1.5
potassium bromide = 1.5
sodium thiosulfite, anhydrate = 1
sodium thiosulfite, pentahydrate = 1
NB: at first it might seem odd that both the penta and anhy of sodium thiosulfite have identical factors, but remember: you need 50% more VOLUME of the penta to make the same strength fixer as the anhydrous)
Yes, jnanian, I could NOT BELIEVE that I could not find it on the Internet. To me, this is utterly impossible given the interest to date. With the WHOLE WORLD on the Internet, this lack is not merely depressing, but unfathomable. (Sorry to wax with such emotion, but WORDS are my 'emoticons', not tawdry symbols.) - David Lyga
And for benefit of the others: possibly these also: (I am largely guessing here and want CONFIRMATION):
glycin = 0.35
borax = 0.9
boric acid powder = 0.9
sodium hydroxide = 0.9
sodium metabisulfite = 1.2
Kodalk = 1.1
phenidone A = 0.45
sodium carbonate, anhy = 0.5
sodium carbonate, mono (washing soda) = 1.17
Also, for further benefit: I REGULARLY divide proprietary powder developers and other powder combinations, (even though I could be hung for doing so). I have NEVER had a problem. NEVER. And I have been doing this for years. If there are any mathemeticians out there I think that they will admit that the Standard Deviation for this is VERY confined. Statistically, especially if the powder is shaken up before measuring volumetrically, the deviation from the theoretical norm (i.e., the actual formula) is very low. Thus, I offer the following volumetric numbers for the milliliters of powder contained within each package:
D-76 gallon size: 290ml (I would imagine that Ilford's ID-11 has the same dry volume)
D-76 replenisher gallon size: 390ml
Dektol gallon size: 465ml
Microdol-X gallon size: 370ml
Kodak Fixer with hardener gallon size: 600ml
Kodak Hypo Clearing Agent gallon size: 330ml (I would also like to know if this powder can substitute for developer preserver, as it is almost entirely sodium sulfite)
Kodak D-11 gallon size: 385ml
Kodak D-19 gallon size: 480ml
Polydol gallon size: 300ml
Polydol replenisher gallon size: 410ml
DK-50 part A gallon size: 24ml
DK-50 part B gallon size: 96ml
DK-50 replenisher part A gallon size: 74ml
DK-50 replenisher part B gallon size: 185ml
Ilford Microphen A US gallon size: 40ml
Ilford Microphen B US gallon size: 280ml
Kodak Selectol Soft gallon size: 180ml
I know that many of these are discontinued, but some people might still have a package or can. If your manufacturer sizes differ from one US Gallon (i.e., like if the size is for one quart or one liter), simply remember that a US Gallon has 3785ml of liquid. - David Lyga
metol = 0.7
hydroquinone = 0.6
sodium sulfite, anhydrate = 1.5
potassium bromide = 1.5
sodium thiosulfite, anhydrate = 1
sodium thiosulfite, pentahydrate = 1
NB: at first it might seem odd that both the penta and anhy of sodium thiosulfite have identical factors, but remember: you need 50% more VOLUME of the penta to make the same strength fixer as the anhydrous)
Yes, jnanian, I could NOT BELIEVE that I could not find it on the Internet. To me, this is utterly impossible given the interest to date. With the WHOLE WORLD on the Internet, this lack is not merely depressing, but unfathomable. (Sorry to wax with such emotion, but WORDS are my 'emoticons', not tawdry symbols.) - David Lyga
And for benefit of the others: possibly these also: (I am largely guessing here and want CONFIRMATION):
glycin = 0.35
borax = 0.9
boric acid powder = 0.9
sodium hydroxide = 0.9
sodium metabisulfite = 1.2
Kodalk = 1.1
phenidone A = 0.45
sodium carbonate, anhy = 0.5
sodium carbonate, mono (washing soda) = 1.17
Also, for further benefit: I REGULARLY divide proprietary powder developers and other powder combinations, (even though I could be hung for doing so). I have NEVER had a problem. NEVER. And I have been doing this for years. If there are any mathemeticians out there I think that they will admit that the Standard Deviation for this is VERY confined. Statistically, especially if the powder is shaken up before measuring volumetrically, the deviation from the theoretical norm (i.e., the actual formula) is very low. Thus, I offer the following volumetric numbers for the milliliters of powder contained within each package:
D-76 gallon size: 290ml (I would imagine that Ilford's ID-11 has the same dry volume)
D-76 replenisher gallon size: 390ml
Dektol gallon size: 465ml
Microdol-X gallon size: 370ml
Kodak Fixer with hardener gallon size: 600ml
Kodak Hypo Clearing Agent gallon size: 330ml (I would also like to know if this powder can substitute for developer preserver, as it is almost entirely sodium sulfite)
Kodak D-11 gallon size: 385ml
Kodak D-19 gallon size: 480ml
Polydol gallon size: 300ml
Polydol replenisher gallon size: 410ml
DK-50 part A gallon size: 24ml
DK-50 part B gallon size: 96ml
DK-50 replenisher part A gallon size: 74ml
DK-50 replenisher part B gallon size: 185ml
Ilford Microphen A US gallon size: 40ml
Ilford Microphen B US gallon size: 280ml
Kodak Selectol Soft gallon size: 180ml
I know that many of these are discontinued, but some people might still have a package or can. If your manufacturer sizes differ from one US Gallon (i.e., like if the size is for one quart or one liter), simply remember that a US Gallon has 3785ml of liquid. - David Lyga
Last edited by a moderator:
- Joined
- Oct 11, 2006
- Messages
- 2,195
- Format
- Multi Format
I am both surprised and pleased that no one has yet posted something like "don't use volume measurements for photo chemicals". The general issue has come up in a few discussions here, and they have usually degenerated into unproductive sniping on either side.
Honestly, I'm not sure I'm understanding the question, but as I wouldn't have an answer, that's irrelevant. 
But I have a question. If one wanted to use D-76 as a one-shot, and let's say they're using a tank that takes 300ml per 35mm roll, would they then use 23ml (22.985ml)? That's also assuming my math is correct?

D-76 gallon size: 290ml (I would imagine that Ilford's ID-11 has the same dry volume)
But I have a question. If one wanted to use D-76 as a one-shot, and let's say they're using a tank that takes 300ml per 35mm roll, would they then use 23ml (22.985ml)? That's also assuming my math is correct?
Presumably the variations in volume due to humidity and compacting of the crystals/powder are so small as to be insignificant? Possibly some things vary more than others, so I was just curious what materials are "good enough" when measured volumetrically.
- Joined
- Mar 6, 2005
- Messages
- 2,261
- Format
- Large Format
I have measured packaged dry chemicals with spoons for 30-35 years and never had a problem.
Examples:
D-76 1+1 = 2 TBS + 15 oz water
I have worked these out for each of the dry chemicals I use through a process of measuring them out. It doesn't take much time or knowledge to do so.
Examples:
D-76 1+1 = 2 TBS + 15 oz water
I have worked these out for each of the dry chemicals I use through a process of measuring them out. It doesn't take much time or knowledge to do so.
Thank you, Wayne. I have the following for these. I wish to CONFIRM the gram/ml factors:
sodium sulfite, anhydrate = 1.5
(big snip)
- David Lyga
I have the following measurements for sodium sulfite. In both cases, the sulfite was shaken down, causing it to settle to a higher density:
Purchased from Photographer's Formulary (in USA): 1.5
Purchased from silverprint.com in the UK: 1.7
Mark Overton
I have the following measurements for sodium sulfite. In both cases, the sulfite was shaken down, causing it to settle to a higher density:
Purchased from Photographer's Formulary (in USA): 1.5
Purchased from silverprint.com in the UK: 1.7
Mark Overton
These data point out the problem with measuring by volume. Since sodium sulfite is usually used in large amounts in BW developers any error for this chemical is not really significant. However it would pose a problem for color developers where it is used in very small amounts.
If you use volume measurement to mix up a developer like D-76 you will get a perfectly workable developer. This assumes that you don't make any errors in the process. However what you get is not quite D-76. IIRC Dr. Henry in his book "Controls in Black and White Photography" discusses the effect of errors in compounding developers. Anyone contemplating using this method should read that portion of his book.
I always enjoyed "Gadget" Gainer's take on those who disallow the possibility of volumetric measurement in his Photo techniques article.
I am both surprised and pleased that no one has yet posted something like "don't use volume measurements for photo chemicals". The general issue has come up in a few discussions here, and they have usually degenerated into unproductive sniping on either side.
That is why I uttered the caveat at the beginning: that I did not wish to be drawn into such nonsense and defamation.
And Kirks518: your math is correct, in that 23ml does indeed equate to making 300ml of FULL STRENGTH D-76, but I am far and away more frugal than that! In fact, I HIGHLY dilute my developers (not for storage, but for immediate use). For a LITER of working solution D-76 I would use only 12ml (!) of powder. But, I first make a stock solution so that I can make only as much working solution as I wish. To make the stock solution (which stores well as long as it is airtight), I mix the 12ml of D-76 in only 100ml of water and add 0.5ml of sodium carbonate, anhydrous to that 100ml. This is my stock solution. To make a working solution, dilute 1+9. Again, this stock solution of 100ml, stores very well (I use tiny 50ml liquor bottles) as it is MORE concentrated than Kodak's stock solution for D-76.
For use (I use about 80F ambient) I dilute that stock solution 1+9 and process something like Ilford PAN F+ for FOUR minutes, Tri-X for about 10 minutes. If you wish to work colder, consider the Fahrenheit factor (for development time change) to be 1.05. In other words, on a calculator, enter 1.05, then multiply by the 4 minutes and press 'equal' for as many degrees Fahr as you wish to lower the temp. You will get the new, increased, development time. For the opposite, to INCREASE temp (from a lower one), enter the 4 minute development time, then divide by your current development time in minutes and press 'equal' for as many times as needed to reflect the degree F change. Remember always to use decimals: 5 and one half minutes is entered as 5.5 and 7 and one quarter minutes is entered as 7.25.
Of course, you can modify the amount of carbonate you add to the stock solution. But do not go overboard or you will end up with paper developer!
DO NOT use higher dilutions than mine or you will get into trouble. Mine is about 6.5X. That is a LOT, but it works well. Consider that to be a practical limit. Use carbonate to adjust the development time to be what you deem most appropriate. - David Lyga
Last edited by a moderator:
I haven't seen any on line, but Anchell, "The Darkroom Cookbook", has quite a list. A few of the more common:
Amidol - 2.33 g/tsp (3.5 per half tbsp)
Hydroquinone - 3 g/tsp
Metol - 3.5 g/tsp
Phenidone - 2 g/tsp
Potassium bromide - 6.4 g/tsp (1.6 per quarter tsp)
Sodium bisulfite (anh) 6 g/ ts[
Sodium carbonate (mono) 6 g/tsp
Sodium metaborate - 4.5 g/tsp
Sodium thiosulfate - 6 g/tsp (18 g/tbsp)
Sodium sulfite (anh) 7.6 g/tsp
These are, of course, approximate. But approximate is good enough for a lot of things, like fixers, and for things where precise or reproducible results are not essential.
Amidol - 2.33 g/tsp (3.5 per half tbsp)
Hydroquinone - 3 g/tsp
Metol - 3.5 g/tsp
Phenidone - 2 g/tsp
Potassium bromide - 6.4 g/tsp (1.6 per quarter tsp)
Sodium bisulfite (anh) 6 g/ ts[
Sodium carbonate (mono) 6 g/tsp
Sodium metaborate - 4.5 g/tsp
Sodium thiosulfate - 6 g/tsp (18 g/tbsp)
Sodium sulfite (anh) 7.6 g/tsp
These are, of course, approximate. But approximate is good enough for a lot of things, like fixers, and for things where precise or reproducible results are not essential.
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |