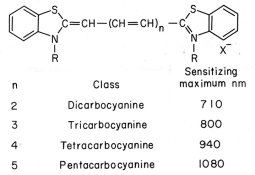
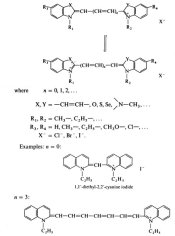
Hello,
I would be very interested in a complete description of how the sensitizing dyes work. Is there a specific mechanism that they all use?
Ben
P.S. Erythrosine seems to have iodine which could be part of the mechanism.
I would be very interested in a complete description of how the sensitizing dyes work. Is there a specific mechanism that they all use?
Ben
P.S. Erythrosine seems to have iodine which could be part of the mechanism.