Photo Engineer
Subscriber
In this part of our discussion we will look at the design of a B&W negative film. Again, it will be done with language as plain as possible with little technical jargon, but I have made some assumptions regarding the readers knowledge of science. I have also simplified much of this for ease of understanding.
The various processes described here can be modeled by computer programs either forward or backward. That is, you can start with the film characteristics and get the required emulsion type or you can start with the emulsion type and get the film design from the characteristics and etc. This model gives the photo engineer a center point for the start of factorial experiments which will lead to the final product. The desired characteristics include toe, shoulder, slope (contrast or gamma), speed (ISO rating), grain and sharpness.
Using the discussion from the last Photo Engineering thread, we know we need a negative film material with an average mid scale gamma (contrast) or slope (change in density / change in exposure) of about 0.6 so lets use that as our average.
Normally, emulsions come in two types. One type is monodisperse (modern style) and the other is polydisperse (early style). Monodisperse means that all of the crystals are the same type and have the same size (approximately) and polydisperse means that all of the crystals have different shapes and sizes. It can be shown (and also be reasoned out logically) that a polydisperse emulsion will have a lower contrast than a monodispersed emulsion. The argument goes thusly: since every grain in a polydisperse emulsion differs, the response of each grain to light varies and gives a different speed for a given exposure. This type of curve is very often bowed due to having some types of grain predominate and therefore this skews the response to light based on a measure called size / frequency distribution. The actual curve may bow upward or downward depending on this size / frequency distribution. This type of emulsion was the only type that could be made in the early years of photographic product manufacturing. It resulted from the making of Single Run (SR) emulsions where Silver Nitrate solution was run into salt and gelatin. Since the salt content varied due to the formation of a precipitate, the type of grain varied from the start of the run until the finish of the run. It is characteristic of many films from the 30s, 40s and 50s. A single emulsion could be tailored to give almost the curve desired, but with sacrifices in mid scale contrast in one way or another. Often, the result was a film with no clear shoulder or a very soft toe.
BTW, for those mathematicians out there, all emulsions, when measured by counting the size of a grain and the frequency they occur in the actual emulsion make up Size Frequency Curves. These curves follow a Gaussian distribution in most cases which is a traditional bell shaped curve. The narrower the curve, the more monodisperse it is and the broader this curve, the more polydisperse. Contrast is related to the width of this curve.
These polydisperse emulsions predominate in most all textbooks up through the first half of the 20th century, particularly those of Wall and those of Baker.
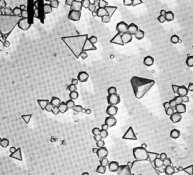
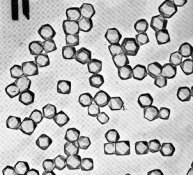
In the attached electron micrographs, the first picture on the left is a polydisperse emulsion with many grain sizes and shapes. It has an inherently low contrast. The middle photomicrograph is a monodisperse emulsion with an octahedral crystalline form. The right photomicrograph shows a cubic emulsion with expitaxy. This exotic emulsion has been first formed as a monodisperse cube and then decorated with the 'ears' on each corner. For more detail see Mees and James, or Haist.
In the 50s and onward, Run Salt (RS) emulsions became the norm and resulted in very straight line curves but high contrast. These emulsions were made by running Silver Nitrate and a separate salt solution into a solution of salt and gelatin. Since the resultant mixture stayed at the same approximate salt concentration from start to finish, the grains were very similar in size and shape. The emulsions made this way were monodisperse. All grains in a monodisperse emulsion reacted pretty much the same to light due to their similarity. The problem was that these emulsions had latitudes that were too short for negative films due to this high contrast. It was found though that by blending such emulsions into one mix and coating them, the result was finer in grain and sharper with a very straight mid scale curve, a sharp toe and a sharp shoulder. In effect, you mixed a fast, medium and slow component. To get an ISO 400 film, the mid scale would be where the correct exposure would be placed and this would be made of an ISO 400 emulsion. The toe would be made up of an ISO 800 emulsion, and the shoulder would be made up of an ISO 200 emulsion. In effect, the starting emulsions coated at silver levels sufficient to achieve a contrast of 1.8 and a Dmax of 3.0 would be coated at 1/3 of the silver level each such that the final silver level of the 3 part blend would be sufficient to achieve a density of 3.0, but the contrasts of each component would be 1/3 the value, and so the result would be a contrast of 0.6, the desired value. This is due to the fact that an emulsion with a contrast of 1.8 and Dmax of 3.0 at say 3000 mg / square meter would have a Dmax of 1.0 if coated at 1000 mg / square meter. The contrast would be about 0.6. Thus the blend of 3 emulsions would result in a film that is the sum of 3 emulsions but with each component coated at 1/3 the level needed to achieve the desired Dmax.
In actual practice, it was found that absorber dyes increased sharpness by reducing internal reflections off of grains. Therefore, in the example above, one might actually use ISO 1600, 800 and 400 emulsions to achieve a mid range of 400. This final, hypothetical film would have much increased sharpness. In fact, at one time, two Kodak products were the same film, but the High Acutance 100 film was merely the 200 film with 1 stop equivalent of acutance dye in it to improve sharpness dramatically. It had the grain of the 200 speed film though.
Design problems cropped up in both early and new type emulsions.
The old type emulsions often were difficult to repeat in terms of curve shape, so the bumps and humps and bows varied from batch to batch. But, the new emulsions did not entirely solve the problem. If the emulsions varied by even a tiny amount in speed or contrast, then a set of 2 bumps arose in the characteristic curve of the film where each emulsion component overlapped. In both types of emulsions (old and new), if the different grains had different makeup such as iodide content, then the grains could equilibrate during keeping in some fashion and this could lead to changes during the life of the film resulting in poor keeping. In the new monodispersed emulsions, it was found that mixing grains with the same composition and placement of chemicals was more optimum, and therefore the modern emulsions kept better.
It can be shown that faster films have higher grain. This is simply due to the cross section of the crystal. The larger the crystal, the more likely it is to catch a photon of light. But there is a tradeoff. The old triangle of characteristics of photographic materials bites us here. That triangle is speed-grain-sharpness. You can have any two of these, but not all 3 from a given film formula. Most often, you only get one of these. So, if you go up in speed, grain gets worse and sharpness may or may not change. Usually though it gets worse. You can increase the level of silver halide that you coat, and sharpness gets a little better up to a point and then turbidity rears its ugly head and you begin to lose speed and sharpness due to internal reflections. Then you must add the acutance dyes, but this causes another speed loss. So you see, a film designer is in a vicious cycle here that can only be resolved by making many coatings of the emulsion(s) with different levels of silver and addenda to optimize speed and grain.
The only way out of this cycle is to find a method by which a given grain size can be kept as is, and the speed of an emulsion can be increased by some other method. This is what is called a speed grain improvement. You get more speed out of a fixed grain size. That is next on the agenda.
The various processes described here can be modeled by computer programs either forward or backward. That is, you can start with the film characteristics and get the required emulsion type or you can start with the emulsion type and get the film design from the characteristics and etc. This model gives the photo engineer a center point for the start of factorial experiments which will lead to the final product. The desired characteristics include toe, shoulder, slope (contrast or gamma), speed (ISO rating), grain and sharpness.
Using the discussion from the last Photo Engineering thread, we know we need a negative film material with an average mid scale gamma (contrast) or slope (change in density / change in exposure) of about 0.6 so lets use that as our average.
Normally, emulsions come in two types. One type is monodisperse (modern style) and the other is polydisperse (early style). Monodisperse means that all of the crystals are the same type and have the same size (approximately) and polydisperse means that all of the crystals have different shapes and sizes. It can be shown (and also be reasoned out logically) that a polydisperse emulsion will have a lower contrast than a monodispersed emulsion. The argument goes thusly: since every grain in a polydisperse emulsion differs, the response of each grain to light varies and gives a different speed for a given exposure. This type of curve is very often bowed due to having some types of grain predominate and therefore this skews the response to light based on a measure called size / frequency distribution. The actual curve may bow upward or downward depending on this size / frequency distribution. This type of emulsion was the only type that could be made in the early years of photographic product manufacturing. It resulted from the making of Single Run (SR) emulsions where Silver Nitrate solution was run into salt and gelatin. Since the salt content varied due to the formation of a precipitate, the type of grain varied from the start of the run until the finish of the run. It is characteristic of many films from the 30s, 40s and 50s. A single emulsion could be tailored to give almost the curve desired, but with sacrifices in mid scale contrast in one way or another. Often, the result was a film with no clear shoulder or a very soft toe.
BTW, for those mathematicians out there, all emulsions, when measured by counting the size of a grain and the frequency they occur in the actual emulsion make up Size Frequency Curves. These curves follow a Gaussian distribution in most cases which is a traditional bell shaped curve. The narrower the curve, the more monodisperse it is and the broader this curve, the more polydisperse. Contrast is related to the width of this curve.
These polydisperse emulsions predominate in most all textbooks up through the first half of the 20th century, particularly those of Wall and those of Baker.
In the attached electron micrographs, the first picture on the left is a polydisperse emulsion with many grain sizes and shapes. It has an inherently low contrast. The middle photomicrograph is a monodisperse emulsion with an octahedral crystalline form. The right photomicrograph shows a cubic emulsion with expitaxy. This exotic emulsion has been first formed as a monodisperse cube and then decorated with the 'ears' on each corner. For more detail see Mees and James, or Haist.
In the 50s and onward, Run Salt (RS) emulsions became the norm and resulted in very straight line curves but high contrast. These emulsions were made by running Silver Nitrate and a separate salt solution into a solution of salt and gelatin. Since the resultant mixture stayed at the same approximate salt concentration from start to finish, the grains were very similar in size and shape. The emulsions made this way were monodisperse. All grains in a monodisperse emulsion reacted pretty much the same to light due to their similarity. The problem was that these emulsions had latitudes that were too short for negative films due to this high contrast. It was found though that by blending such emulsions into one mix and coating them, the result was finer in grain and sharper with a very straight mid scale curve, a sharp toe and a sharp shoulder. In effect, you mixed a fast, medium and slow component. To get an ISO 400 film, the mid scale would be where the correct exposure would be placed and this would be made of an ISO 400 emulsion. The toe would be made up of an ISO 800 emulsion, and the shoulder would be made up of an ISO 200 emulsion. In effect, the starting emulsions coated at silver levels sufficient to achieve a contrast of 1.8 and a Dmax of 3.0 would be coated at 1/3 of the silver level each such that the final silver level of the 3 part blend would be sufficient to achieve a density of 3.0, but the contrasts of each component would be 1/3 the value, and so the result would be a contrast of 0.6, the desired value. This is due to the fact that an emulsion with a contrast of 1.8 and Dmax of 3.0 at say 3000 mg / square meter would have a Dmax of 1.0 if coated at 1000 mg / square meter. The contrast would be about 0.6. Thus the blend of 3 emulsions would result in a film that is the sum of 3 emulsions but with each component coated at 1/3 the level needed to achieve the desired Dmax.
In actual practice, it was found that absorber dyes increased sharpness by reducing internal reflections off of grains. Therefore, in the example above, one might actually use ISO 1600, 800 and 400 emulsions to achieve a mid range of 400. This final, hypothetical film would have much increased sharpness. In fact, at one time, two Kodak products were the same film, but the High Acutance 100 film was merely the 200 film with 1 stop equivalent of acutance dye in it to improve sharpness dramatically. It had the grain of the 200 speed film though.
Design problems cropped up in both early and new type emulsions.
The old type emulsions often were difficult to repeat in terms of curve shape, so the bumps and humps and bows varied from batch to batch. But, the new emulsions did not entirely solve the problem. If the emulsions varied by even a tiny amount in speed or contrast, then a set of 2 bumps arose in the characteristic curve of the film where each emulsion component overlapped. In both types of emulsions (old and new), if the different grains had different makeup such as iodide content, then the grains could equilibrate during keeping in some fashion and this could lead to changes during the life of the film resulting in poor keeping. In the new monodispersed emulsions, it was found that mixing grains with the same composition and placement of chemicals was more optimum, and therefore the modern emulsions kept better.
It can be shown that faster films have higher grain. This is simply due to the cross section of the crystal. The larger the crystal, the more likely it is to catch a photon of light. But there is a tradeoff. The old triangle of characteristics of photographic materials bites us here. That triangle is speed-grain-sharpness. You can have any two of these, but not all 3 from a given film formula. Most often, you only get one of these. So, if you go up in speed, grain gets worse and sharpness may or may not change. Usually though it gets worse. You can increase the level of silver halide that you coat, and sharpness gets a little better up to a point and then turbidity rears its ugly head and you begin to lose speed and sharpness due to internal reflections. Then you must add the acutance dyes, but this causes another speed loss. So you see, a film designer is in a vicious cycle here that can only be resolved by making many coatings of the emulsion(s) with different levels of silver and addenda to optimize speed and grain.
The only way out of this cycle is to find a method by which a given grain size can be kept as is, and the speed of an emulsion can be increased by some other method. This is what is called a speed grain improvement. You get more speed out of a fixed grain size. That is next on the agenda.