(or this one weird trick....)
I didn't know where best to post this. But since I fell into this “discovery” (didn't find any instances in literature, do point out otherwise) while working on my salt print process, this forum might be as good as any. It seems that the Himalayan Black (or Pink) Salt (you would know it if you know South Asian cuisine well as I do) can act as a pretty good (?) toner for silver prints.
Recently I decided to experiment with Polysulfide (Kodak Brown Toner or T-8 type) toning to see for myself if anything good happens with salt prints. Both selenium and sulfide toning are not as popular with salt printers as various gold/platinum/palladium based toners, due to their propensity to severely bleach the images. In any case, I acquired some liver of sulfur for that purpose and soon as I opened the bottle, the smell reminded me (among other things) of the
black salt. For those who don't know,
Black salt or Kala Namak (literal translation in Hindi)
(
https://en.wikipedia.org/wiki/Kala_namak)
is a natural salt mined along the Himalayas. It contains loads of minerals as well as many sulfurous compounds including sulfides, sulfites etc. Turns out I had a some in my spice cabinet so I thought let's see what would happen if I make a solution and tested it for toning.
I took a few test strips of salt print (ironically, salt-free salt print - details of which I will present in another thread at some point) all fixed and washed and dunked them in various concentrations of
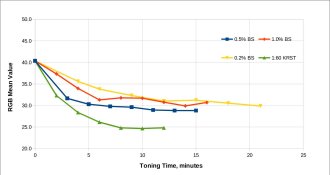
black salt 0.2% to 5% in plain water (no other additives) for 10 minutes each.
Attached files summarize the results. Each strip contained two zones for 40 minutes and 5 minutes each of UV box exposure – signifying 3 stops of difference. Toning (which I define as increase in density and/or change in color towards more neutral) was achieved to varying degree accompanied by bleaching (loss of density) as well, particularly in the lighter step. As the
black salt concentration went up, the tone changed towards more neutral from the reddish brown/yellow hues of the control un-toned sample. That was evident even at lowest 0.2% concentration. The darker 40 minutes step intensified in all cases except the 5% one. In the lighter zoneS, there was bleaching at all concentrations, worst being at the highest. In essence, if one considers the delta between the 40 min and 5 min exposureS (Column 4 in the spread sheet) as some sort of contrast measure, it improves with toner concentration, a fact that can be used in one's favor if using lower contrast negatives.
Conclusion:
I might have stumbled upon yet another way to tone the salted prints using the Himalayan black salt. Granted this is not a very well defined as a chemical and the efficacy will vary depending on where it comes from, but it is very cheap and edible to the boot. Nowadays it is available practically everywhere - even Walmart sells it. It does give off smell but in a well ventilated area, there should be no problem.
More optimizing needs to be done. The 0.2-1% toner concentration look the most promising in terms of maximizing the color shift with modest Dmax boost and least bleaching at lower density. More time dependent experiments will help. Perhaps addition of an alkali like sodium carbonate may influence the bleaching behavior as well.
Much to do....
Feel free to try out yourselves!
:Niranjan.
 I have tried Liver of Sulfur, another favorite of jewellery artists (which is also the basis of polysulfide toner) and so far the results have been analogous to the black salt. If you are suggesting use of bare H2S gas, synthesized somehow in-situ and exposing the print, I would be careful as it is toxic if over-exposed to - not something I would like to play around with.
I have tried Liver of Sulfur, another favorite of jewellery artists (which is also the basis of polysulfide toner) and so far the results have been analogous to the black salt. If you are suggesting use of bare H2S gas, synthesized somehow in-situ and exposing the print, I would be careful as it is toxic if over-exposed to - not something I would like to play around with.