This probably sits halfway between alt process and b&w chemistry really ... thought it might be of interest, perhaps even might trigger some technical speculation by the Chymists.
Anyway ... I have been experimenting with making Iron Gall Ink for a friend. The first step is to crush some oak galls and set them to ferment in rainwater in a jar for a couple of months.
(The theory seems to be - if I am paraphrasing accurately - that the combination of hydrolysis and enzyme action from fermentation will turn some or much of the Tannic acids and Gallotannic acids in the galls to Gallic acid; the subsequent step is to add Ferrous sulfate to the mixture, which reacts with the gallic acid present to form a pigment, which is the basis of the ink. IG ink is extremely robust once on paper, and does not fade)
Now, from my (dangerously superficial) understanding of photochemistry I know that Gallic acid can be used as a toner for cyanotypes and other alt-processes, and that it is a precursor for the production of Pyrogallic acid, which (as any skoolboy kno) is a developing agent for Silver halides.
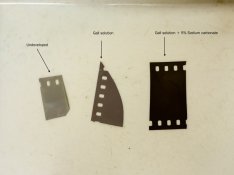
SO, thought I, let's dunk a bit of exposed 35mm leader in some of this fermenting goo for an hour, just to see what happens. Results in attachment.
Clearly something's gone on ... but of course we know that all sorts of macerated plant materials with a bit of alkali added will develop film, so there's no conclusion as to what the developing agent is here.
Anyone interested in IG ink might want to have a look at this rather beautiful little site, by the way: www.irongallink.org
Anyway ... I have been experimenting with making Iron Gall Ink for a friend. The first step is to crush some oak galls and set them to ferment in rainwater in a jar for a couple of months.
(The theory seems to be - if I am paraphrasing accurately - that the combination of hydrolysis and enzyme action from fermentation will turn some or much of the Tannic acids and Gallotannic acids in the galls to Gallic acid; the subsequent step is to add Ferrous sulfate to the mixture, which reacts with the gallic acid present to form a pigment, which is the basis of the ink. IG ink is extremely robust once on paper, and does not fade)
Now, from my (dangerously superficial) understanding of photochemistry I know that Gallic acid can be used as a toner for cyanotypes and other alt-processes, and that it is a precursor for the production of Pyrogallic acid, which (as any skoolboy kno) is a developing agent for Silver halides.
SO, thought I, let's dunk a bit of exposed 35mm leader in some of this fermenting goo for an hour, just to see what happens. Results in attachment.
Clearly something's gone on ... but of course we know that all sorts of macerated plant materials with a bit of alkali added will develop film, so there's no conclusion as to what the developing agent is here.
Anyone interested in IG ink might want to have a look at this rather beautiful little site, by the way: www.irongallink.org
Attachments
Last edited by a moderator:



 The extract may stain emulsions.
The extract may stain emulsions. )
)