nmp
Member
The Background:
Developed-out salted paper prints go as far back as 1850’s, credited to Louis Désiré Blanquart-Evrard. Typically it involved a short exposure forming a hint of an image on salted paper followed by development with gallic acid and some silver nitrate to facilitate the physical development.
The main advantage of such a process as opposed to the POP salted paper is that an image can be printed with much lower UV exposure - so at the time there were no artificial sources of UV light, printing could be accomplished on the a cloudy day when it otherwise would take hours of exposure.
More recently, there is a renewed interest among some who would like to make a print directly from an enlarger fitted with a UV source where exposures for a POP salt can be very long. If somehow the times can be cut down, it would be much more viable to go straight from an analogue negative to an enlarged print. This can also perhaps be useful in making in-camera negatives in the same fashion as calotypes, which also involves physical development with gallic acid. Some of these issues were discussed in a thread started by @NedL. There I shared an idea using a Vitamin C developer after removing excess silver nitrate from the paper by washing with distilled water so the development is strictly of the chemical kind and not the physical kind or a combination of both.
Well, that was over 3 years ago and finally I had a chance to try that out. In this particular case, instead of washing out the silver nitrate remaining in the paper as I had suggested then (why waste perfectly good silver,) I treat the paper with 5% NaCl, the same way as one would do in the traditional salt process. What I found is that at that concentration of salt, practically all of the silver chloride formed stays in the paper. There is no wash-out (as confirmed by no action on the affluent with exposure to sunlight or on addition of selenium toner) of any silver chloride as one would see when tap water is used. So now we have a paper with 100% silver chloride, not unlike a silver gelatin paper and a faint image from a short exposure beforehand.
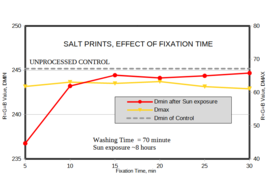
Next comes development with a suitable developer, in this case one containing ascorbic acid or Vitamin C, followed by plain water stop bath, fix, etc – essentially same steps as the salted paper process. As is typical with any new process or for that matter any alternative process one is trying to learn, the challenge is how to get darkest shadows without staining or fogging in the areas that received no exposure and all the tonal variations in-between. After some trial and error, I have found a starter set of exposure time, developer composition/time that gives good whites as well as decent Dmax.
The process is detailed in the next post.
:Niranjan.
Developed-out salted paper prints go as far back as 1850’s, credited to Louis Désiré Blanquart-Evrard. Typically it involved a short exposure forming a hint of an image on salted paper followed by development with gallic acid and some silver nitrate to facilitate the physical development.
The main advantage of such a process as opposed to the POP salted paper is that an image can be printed with much lower UV exposure - so at the time there were no artificial sources of UV light, printing could be accomplished on the a cloudy day when it otherwise would take hours of exposure.
More recently, there is a renewed interest among some who would like to make a print directly from an enlarger fitted with a UV source where exposures for a POP salt can be very long. If somehow the times can be cut down, it would be much more viable to go straight from an analogue negative to an enlarged print. This can also perhaps be useful in making in-camera negatives in the same fashion as calotypes, which also involves physical development with gallic acid. Some of these issues were discussed in a thread started by @NedL. There I shared an idea using a Vitamin C developer after removing excess silver nitrate from the paper by washing with distilled water so the development is strictly of the chemical kind and not the physical kind or a combination of both.
Well, that was over 3 years ago and finally I had a chance to try that out. In this particular case, instead of washing out the silver nitrate remaining in the paper as I had suggested then (why waste perfectly good silver,) I treat the paper with 5% NaCl, the same way as one would do in the traditional salt process. What I found is that at that concentration of salt, practically all of the silver chloride formed stays in the paper. There is no wash-out (as confirmed by no action on the affluent with exposure to sunlight or on addition of selenium toner) of any silver chloride as one would see when tap water is used. So now we have a paper with 100% silver chloride, not unlike a silver gelatin paper and a faint image from a short exposure beforehand.
Next comes development with a suitable developer, in this case one containing ascorbic acid or Vitamin C, followed by plain water stop bath, fix, etc – essentially same steps as the salted paper process. As is typical with any new process or for that matter any alternative process one is trying to learn, the challenge is how to get darkest shadows without staining or fogging in the areas that received no exposure and all the tonal variations in-between. After some trial and error, I have found a starter set of exposure time, developer composition/time that gives good whites as well as decent Dmax.
The process is detailed in the next post.
:Niranjan.
Last edited:








 I am planning to retire pretty soon...
I am planning to retire pretty soon...