Please don't be unhappy with me, I am not trying to destroy APUG by bringing up "grain clumping", but I have a question I'd like to find out about. I tried not to give this thread a clickbait title.
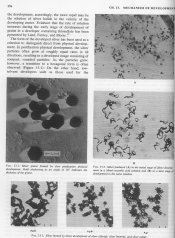
I understand that AgX crystals are fixed in place and cannot migrate in an emulsion, however I was just reading Kurt Jacobsen's book "Developing" (18th ed.) and on page 53 there is a diagram and explanation of what he calls grain clumping -- where the reduced silver between two adjacent AgX grains that are developing can form a bridge between each other, thus forming a single, irregularly shape large silver grain. Is this notion of "grain clumping" also incorrect? I'd be interested to learn more about it. I don't take Jacobsen's book to be an absolute authority on photographic science (such as Haist's or Mees'), but I was under the impression that his book was fairly reputable (at least the later editions). Has the meaning of grain clumping changed over the years from "an actually occurring process of discrete adjacent AgX crystals developing into a larger irregular mass of silver" to "a fictional process whereby AgX crystals can move throughout the emulsion"?
If adjacent developing AgX crystals can form a larger agglomerate of silver, can solvent developers prevent this from happening, and if so what is the mechanism?
I have more questions regarding covering power of developed masses of silver, and the effect of physical versus chemical development on this, but I'll try not to complicate things all at once.
Cheers,
Ian
I understand that AgX crystals are fixed in place and cannot migrate in an emulsion, however I was just reading Kurt Jacobsen's book "Developing" (18th ed.) and on page 53 there is a diagram and explanation of what he calls grain clumping -- where the reduced silver between two adjacent AgX grains that are developing can form a bridge between each other, thus forming a single, irregularly shape large silver grain. Is this notion of "grain clumping" also incorrect? I'd be interested to learn more about it. I don't take Jacobsen's book to be an absolute authority on photographic science (such as Haist's or Mees'), but I was under the impression that his book was fairly reputable (at least the later editions). Has the meaning of grain clumping changed over the years from "an actually occurring process of discrete adjacent AgX crystals developing into a larger irregular mass of silver" to "a fictional process whereby AgX crystals can move throughout the emulsion"?
If adjacent developing AgX crystals can form a larger agglomerate of silver, can solvent developers prevent this from happening, and if so what is the mechanism?
I have more questions regarding covering power of developed masses of silver, and the effect of physical versus chemical development on this, but I'll try not to complicate things all at once.
Cheers,
Ian