Hi everybody, I was tinkering with the peroxide/vinegar bleach and had some weird results. (BTW I wasnt trying to do any reversal, I was trying to work something else out).
"bleach" was at 40C
The first solution was a 2/1 3%peroxide/10%vinegar the result was nil for 10 mins however I picked it out with the tweezers and in a short time from solution to wash under the tap it left a mark:


I progressively added peroxide to a 4/1 ratio and even that gave no apparent reaction in the immersed part but the part I was holding started to look a bit discolored (was using my fingers not the tweezers so I'm sure the top side didn't get wet), again went to wash and got worse:

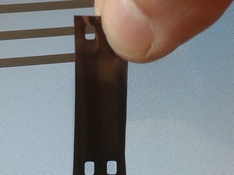
Tested a piece of old negative (found film and I messed up putting in the reel so was sticking to itself when I developed it ages ago, I use the pieces for this kind of stuff) added peroxide to the solution until I saw just a couple of random bubbles from the film
5 minutes in total, no change but 5 or 6 bubbles, took it out the solution to look at it better against the light and it started turning, again kept going while I washed it:

The tan part is where the film was sticking, the middle had room and developed properly, got quite dark and the developed image its blotted out save for a small sliver, visible with strong light.
The seethrough areas shown up outside the bleach while I looked at it and during the wash.
A solution 1/16 3%peroxide/5%vinegar like the internet examples for reversal cleared a piece of film in less than 30 seconds, and ruined the emulsion in the process but in this one it happened to the part immersed in the solution at least

What I do not understand is why the film started clearing when outside the bleach and nothing was happening while it was sitting in it and why so unevenly (beside the last one of which the only surprise was the speed)
I'm baffled most of all by the one I was holding with the fingers because the discolored area didn't even get at all in the "kind of bleach" solution.
"bleach" was at 40C
The first solution was a 2/1 3%peroxide/10%vinegar the result was nil for 10 mins however I picked it out with the tweezers and in a short time from solution to wash under the tap it left a mark:


I progressively added peroxide to a 4/1 ratio and even that gave no apparent reaction in the immersed part but the part I was holding started to look a bit discolored (was using my fingers not the tweezers so I'm sure the top side didn't get wet), again went to wash and got worse:


Tested a piece of old negative (found film and I messed up putting in the reel so was sticking to itself when I developed it ages ago, I use the pieces for this kind of stuff) added peroxide to the solution until I saw just a couple of random bubbles from the film
5 minutes in total, no change but 5 or 6 bubbles, took it out the solution to look at it better against the light and it started turning, again kept going while I washed it:

The tan part is where the film was sticking, the middle had room and developed properly, got quite dark and the developed image its blotted out save for a small sliver, visible with strong light.
The seethrough areas shown up outside the bleach while I looked at it and during the wash.
A solution 1/16 3%peroxide/5%vinegar like the internet examples for reversal cleared a piece of film in less than 30 seconds, and ruined the emulsion in the process but in this one it happened to the part immersed in the solution at least

What I do not understand is why the film started clearing when outside the bleach and nothing was happening while it was sitting in it and why so unevenly (beside the last one of which the only surprise was the speed)
I'm baffled most of all by the one I was holding with the fingers because the discolored area didn't even get at all in the "kind of bleach" solution.
Last edited:


