- Joined
- Nov 16, 2004
- Messages
- 3,384
I made up 3 developers as given in the following formula,except that as developing agent one had sodium ascorbate only,the second had pyrocatechin only and the third was as given.All had 5g Carbonate.
Sodium Ascorbate.......................10g
Pyrocatechin...............................0.2g
Sodium Carbonate anh...................5g
Water to.....................................1L
Fomapan 400 was developed 12m 21C ag 10s/min.Resulting negatives are shown in fig 1 attached.
With sodium ascorbate alone there was insignificant development.
Pyrocatechin alone gave a contrasty image with tanning of the emulsion (verified by bleaching in ferricyanide/bromide and fixing to give the relief image).
Pyrocatechin plus ascorbate gave the densest image.There was no tanning.
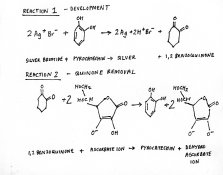
Referring to fig 2,reaction 1 explains development by pyrocatechin.It is the quinone that causes tanning.
How to explain the greater density and lack of tanning with pyrocatechin plus ascorbate?
My explanation is that lack of tanning is due to absence of quinone.This also increases the density by driving reaction 1 to the right.Absence of quinone is attributed to its reaction with ascorbate .It is regenerated to pyrocatechin.Ascorbate is converted to dehydroascorbate.This is shown as reaction 2,fig 2.
Thanks for comment.
Sodium Ascorbate.......................10g
Pyrocatechin...............................0.2g
Sodium Carbonate anh...................5g
Water to.....................................1L
Fomapan 400 was developed 12m 21C ag 10s/min.Resulting negatives are shown in fig 1 attached.
With sodium ascorbate alone there was insignificant development.
Pyrocatechin alone gave a contrasty image with tanning of the emulsion (verified by bleaching in ferricyanide/bromide and fixing to give the relief image).
Pyrocatechin plus ascorbate gave the densest image.There was no tanning.
Referring to fig 2,reaction 1 explains development by pyrocatechin.It is the quinone that causes tanning.
How to explain the greater density and lack of tanning with pyrocatechin plus ascorbate?
My explanation is that lack of tanning is due to absence of quinone.This also increases the density by driving reaction 1 to the right.Absence of quinone is attributed to its reaction with ascorbate .It is regenerated to pyrocatechin.Ascorbate is converted to dehydroascorbate.This is shown as reaction 2,fig 2.
Thanks for comment.